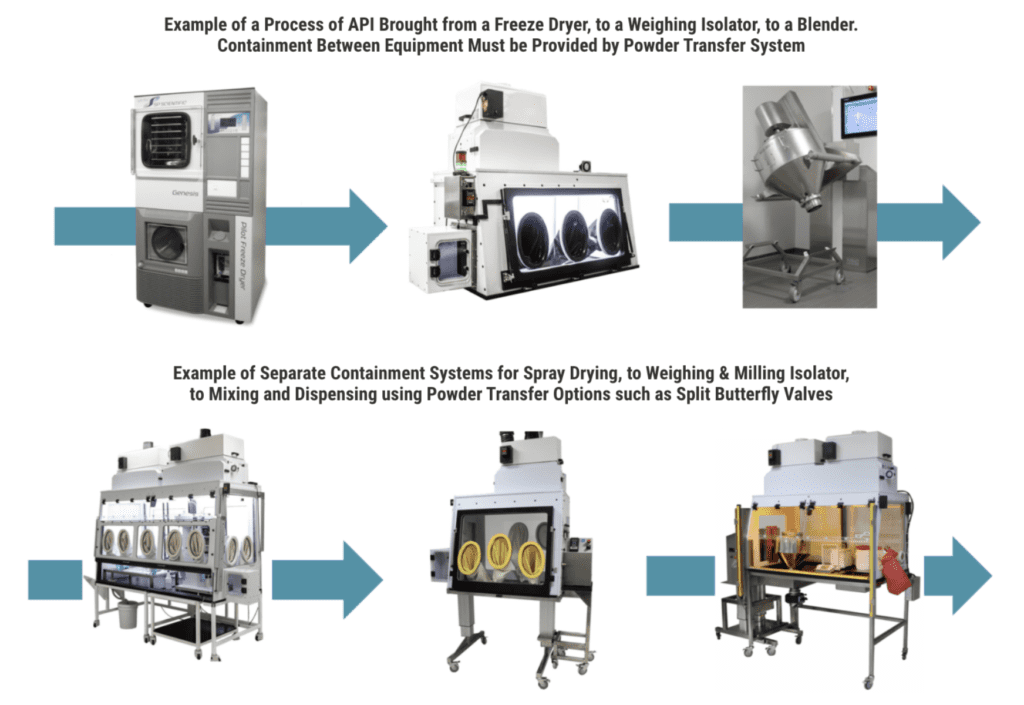
POWDER TRANSFER OPTIONS
In pharmaceutical manufacturing, applications that use a continuous process allow for transfer through containment. It is important for Flow Sciences to understand during the engineering and planning phase where the hazardous material is coming from and how it is entering the containment system, as well as how it is exiting and where it is going. …