

STERILE & NON-STERILE GLOVE BOXES AND CONTAINMENT SYSTEMS FOR HPAPI PROCESSING

• WEIGHING / HPAPI SYNTHESIS
• CONJUGATION & PURIFICATION
• LYOPHILIZATION AND FILLING
• DRYING & COMPRESSION
• MULTI-TASK SYSTEMS
Particle Containment
Engineering controls designed to mitigate exposure and create separation between the operator and the toxic material are available in many different forms, all with their own unique advantages and risks. Depending on application, toxicity, equipment, and other factors, operating in an enclosure or glovebox for particle containment provides personnel protection, product protection, or both.
ADC Manufacturing
Manufacturing antibody-drug conjugates (ADCs) poses unique challenges at each stage of processing—from initial HPAPI manipulation all the way to patient testing. ADC manufacturing facilities require major initial investment and extensive operator training. The weighing and dissolution of HPAPI, considered the most hazardous step, must be carried out in a negative pressure enclosure before it is transferred into a sterile environment. Once the HPAPI is weighed and dissolved in to a solution, it is slightly safer to handle—though it still must be channeled through a series of engineering controls, often spread over the space of several labs.
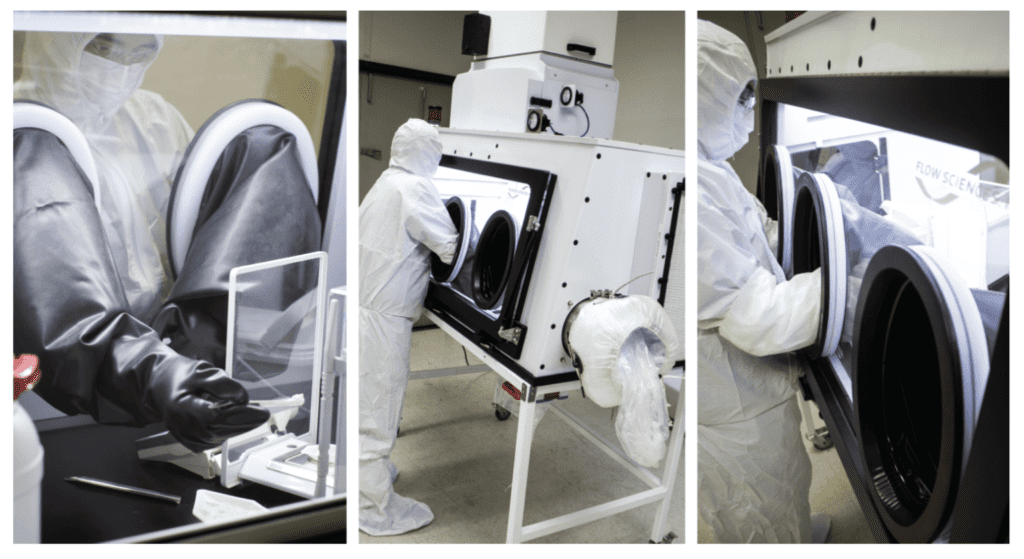
Cytotoxic Glovebox Workstation
Flow Sciences’ glovebox workstations have long been lauded for personnel and product protection, as well as exceeding containment performance and targets when dealing with even the most highly potent APIs. The Glovebox Workstation offers sub-nanogram containment, which is critical for ADC applications given the potency of materials. Decontamination has always been a concern for labs working on ADC— this rigid wall solution allows for companies to utilize better equipment that won’t be destroyed after a single use, and let manufacturers integrate enhanced washdown/cleaning protocols to satisfy increasing scrutiny on proper safe handling of conjugates. The Glovebox Workstation is the first step in an end-to-end process for research and development markets that are growing exponentially.
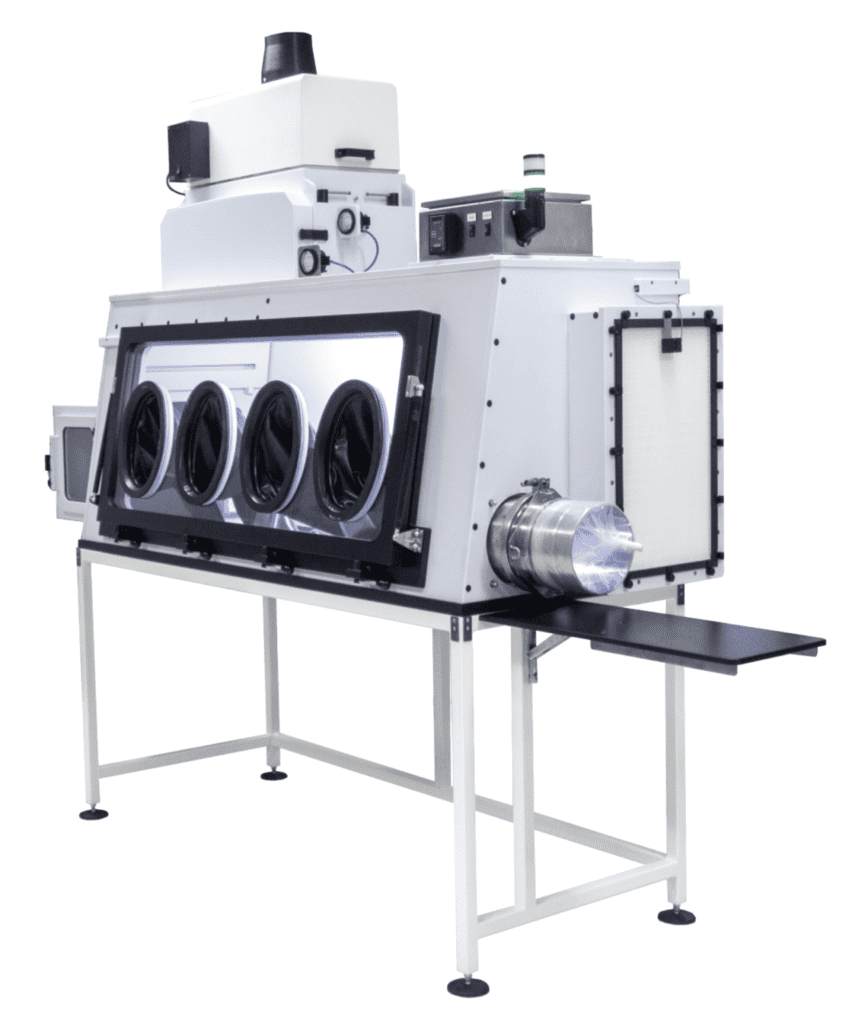
Standard Features
• DESIGNED FOR HPAPI MANIPULATION
• ISO 5 OR BETTER INTERIOR CLEANLINESS
• NEGATIVE PRESSURE HEPA FILTRATION
• GLASS OR ACRYLIC VIEWING PANELS
• MANY BASE (WORKTOP) OPTIONS
• STANDARD 4’, 5’, AND 6’ WIDTHS
• 6-7 PLACE BALANCE STABILITY
• UNIVERSAL SIDE PORT / TRANSFER PORT
• WITH OR WITHOUT STAND
Optional Features
• CUSTOM SIZING AND CONFIGURATION
• STAINLESS STEEL BASE OR SUPERSTRUCTURE
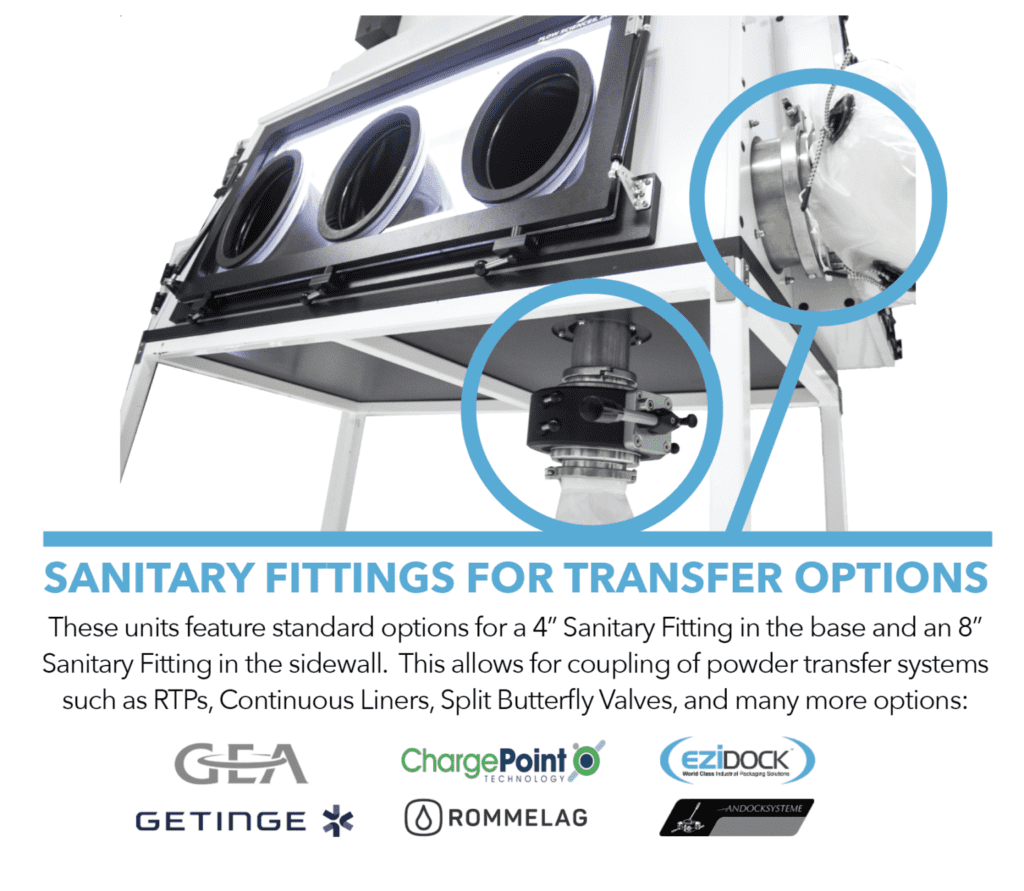
• CLEAN IN PLACE (CIP) & WASHDOWN
• JOINING OF OTHER CONTAINMENT SYSTEMS
• VACUUM AND OTHER SERVICE INTEGRATION
CONTAINMENT SOLUTIONS
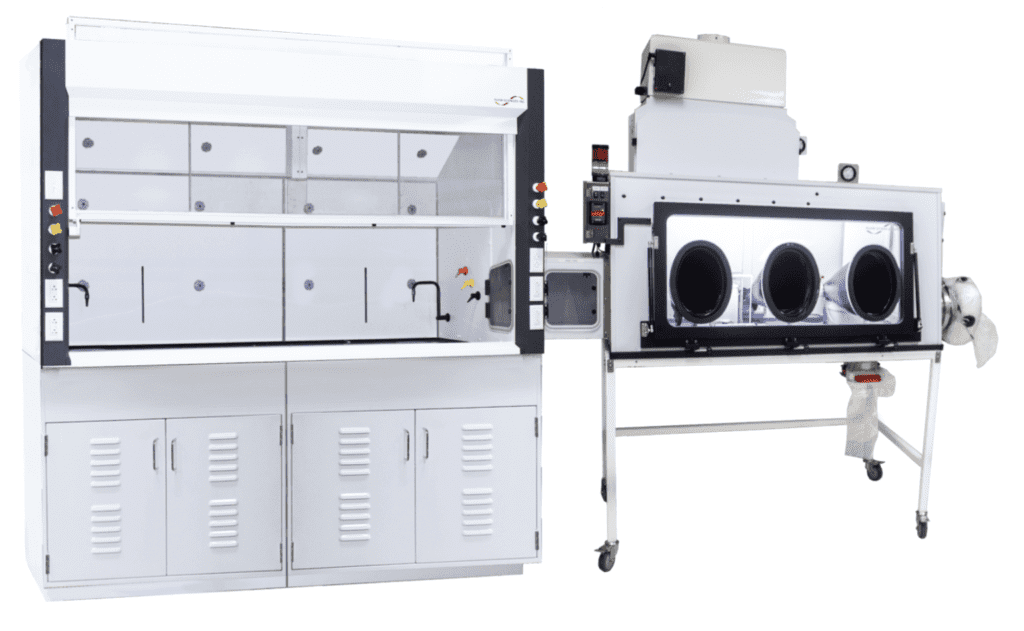
War Head Synthesis
• Chemical Fume Hoods Connected to Glovebox Workstations for High Potency API handling to solution
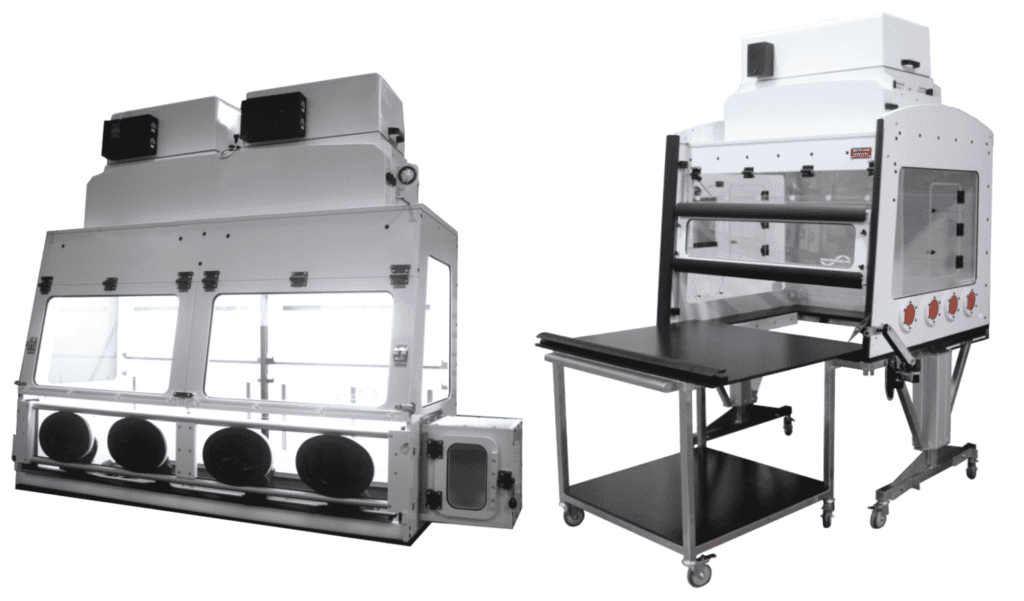
Conjugation & Purification - Non-Sterile
Externally Vented Oversized Enclosures for Lab Equipment Including:
• Laser Particle Analysis
• HPLC Flash Chromatography
• Laser Diffraction
• Other Lab Process Equipment

Sterile Conjugation
The Microsphere provides a physical barrier between product and operator which eliminates the need for a cleanroom while conjugating. During ADC processing, this is where the antibody is purified to remove process-related contaminants (unconjugated toxin and residual solvent).
• ISO 4 in the main chamber & ISO 5 in the antechamber
• Cost efficient unidirectional positive pressure C.A.I.
• Recovery rate of less than 60 seconds
• Ease of use, ergonomic, easy to install, maintain, and operate
• Optional TSPs : Total Service Plans that include certification, SOPs, and
essentials
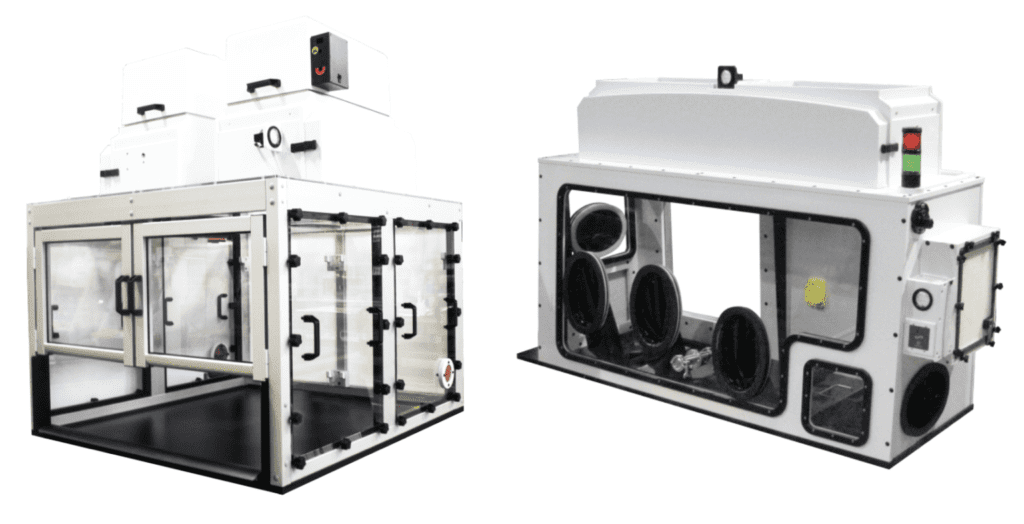
Lyophilization & Filling - Non-Sterile
• Benchtop Lyophilizer Containment Systems with Iris Ports
• Glovebox Workstations conjoined with Lyophilizers and Biological Safety Cabinets to introduce trays from freeze dryer directly into containment
• Other Lab Process Equipment

Aseptic Formulation
The Chemosphere allows for additional processing since ADCs for human clinical
applications must be manufactured in an aseptic biological environment that operates according to cGMP. The formulation of the ADC takes place at this step.
• ISO Class 4 in the main chamber & ISO Class 5 in the antechamber
• Unidirectional and negative pressure C.A.C.I
• Alarms and interlocks for prevention and safety
• Fast recovery rate (less than 1 minute)
• Ease of use, ergonomic, easy to install, maintain, and operate
• Optional TSPs : Total Service Plans that include certification, SOPs, and
essentials
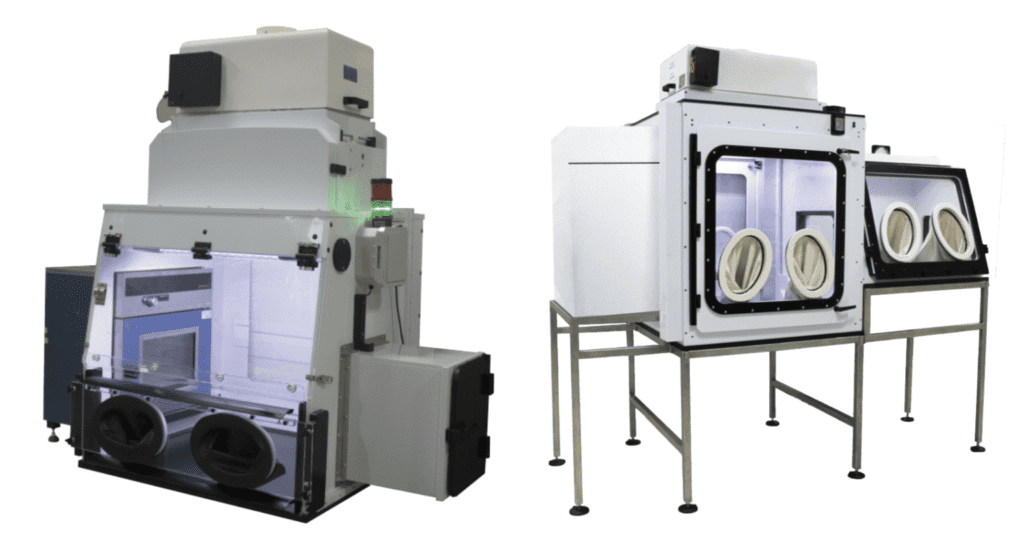
Drying & Compression
• Safe localized containment for ovens and other lab equipment
• Integration ovens or through the sidewall or rear of the enclosure

Aseptic Filling / Sterility Testing
The Isotech Isophere Sterility Tester allows for decontamination of the ADC which utilizing H202, which overcomes the problems associated with conventional surface disinfection validation. It is safer while being less expensive than cleanroom alternatives.
• ISO 4 unidirectional airflow
• Eliminates the risk of high start-up costs, extended deadlines, and possible
contamination
• Complete product/operator protection
• Small critical area: increased SAL
• Minimal energy requirements
• Filling equipment integration, interface with lyophilizers, autoclaves and DHS
• Decontamination with H2O2 generator
• IQ/OQ validation: documentation & execution
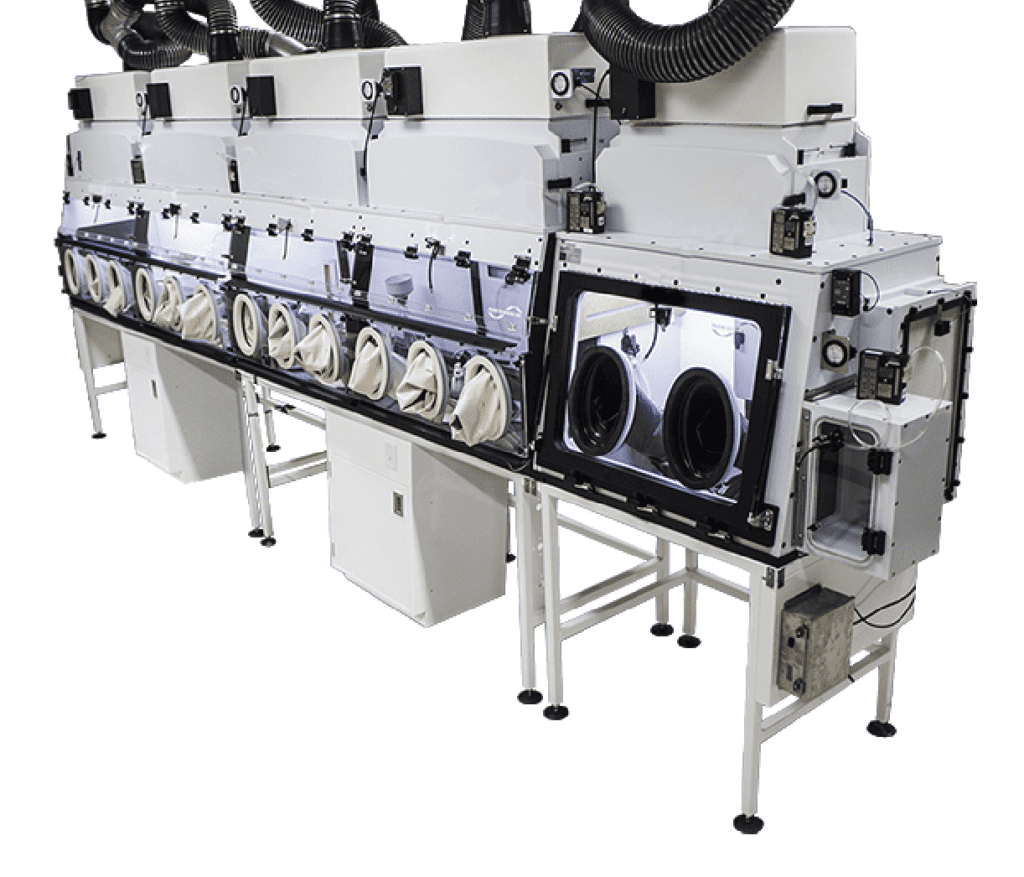
Multi-Task Systems - Conjoined Systems
• Integrations of various equipment types in a contained process line
• Various options of enclosure styles for connecting and joining

