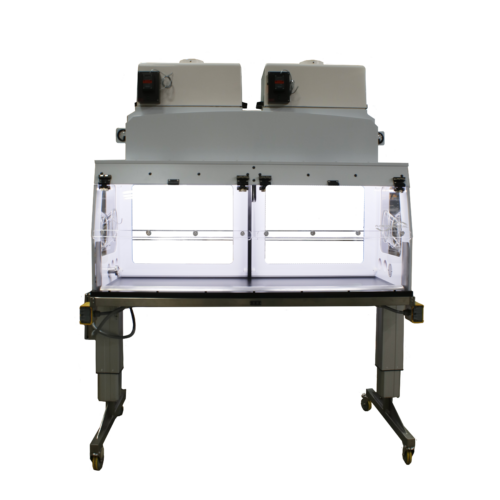
Application: Powder Weighing and Dispensing with Getinge La Calhene Alpha-Beta Ports
This stainless steel enclosure is designed for powder dispensing applications for facilities performing powdered Highly Potent Active Pharmaceutical Ingredient (HPAPI) weighing and dispensing operations. Particularly, it was designed for operations conducted in facilities operating under the stipulations of current Good Manufacturing Practices (cGMPs). The working space allows operators to freely conduct the operation by weighing a large batch (100 grams or more) of powder, dispensing the powder into a container, sealing the container, and cleaning the enclosure after use. Additionally, two ball valve fittings (3/8” NPT) are located on the right side of the enclosure for connection to inert gas sources. This connection is advantageous for sample protection by facilitating dehumidification and deoxidization of the sample environment for powder substances with attributes incurring the need for inert gas (e.g. pyrophoric powders, hygroscopic powders, high reactivity with oxygen, etc.).
After filling the bag with powder, the operator has the capability of moving the bagged powder from the enclosure to another enclosure with protection from exposure during transport. This function is made possible through the usage of an integrated Alpha Getinge La Calhene Rapid Transfer Port (RTP) on the right side of the enclosure. The incorporation of the Alpha RTP facilitates safe transfer by allowing the attachment of a Beta RTP conjugate capsule to the Alpha RTP. Following attachment, the operator is able to transfer the desired amount of powder (or aqueous solution*) from the enclosure interior, through an opening created by the Alpha/Beta connection, and into a Beta RTP capsule. From here, both the Alpha and Beta conjugates are sealed and the Beta capsule is used as a transport vehicle to the other enclosure. Referring back to the previous paragraph, the enclosure also allows for the transfer process occur, in reverse, after the Beta capsule is transported to the next enclosure or RTP-compatible device.
When designing the enclosure, Flow Sciences also considered ease and efficiency of process flow. Thus, its interior layout accommodates space for 1-2 analytical balances as well as sufficient working space around each balance for safe and effective use during your operation. Specifically, the operator is provided with ample room to move their arms to weigh, removed weighed powder, and dispense the powder into the bag.
After Factory Acceptance Testing (FAT), the enclosure was proven to contain down to an 8-hour Time Weighted Average (TWA) concentration of 100 nanograms per cubic meter of air (ng/m3). The actual level of containment was proven to be 0.06 ng/m3.

“Containment Target”, as depicted in the image above, is the respiratory exposure concentration specified by the customer. The “Surrogate Powder Testing Result” is the actual exposure concentration result from air samples taken during performance validation testing conducted by FSI. The surrogate “contaminant” sampled during the FAT was a powder substance with attributes similar to that of the actual contaminant.
When designing the enclosure, Flow Sciences also considered ease and efficiency of process flow. Thus, its interior layout accommodates space for the Hydro SV and the Mastersizer 3000. Specifically, the operator is provided with ample room to move their arms to fill the cuvette, insert the cuvette into the Mastersizer 3000, and perform analysis; all while retaining space for wiring connections.
*Note:If the Occupational Exposure Limit (OEL) or Occupational Exposure Band (OEB) for the pertinent HPAPI contaminant(s) are lower than 1 microgram per cubic meter of air (1 ug/m3), dissolution of the sample into an aqueous solution is an alternative method to reduce the risk of overexposure during RTP transport.