- All
- Literature
- Papers
- Press Releases
- Videos
- Webinars
Introducing Our Technical Support Division
Flow Sciences, Inc., a leading provider of containment solutions for laboratories and industrial facilities, has announced the launch of its new Technical Support Department, led by Karl Yeager. This initiative emphasizes the company’s commitment to exceptional after-sales service and support. Under Yeager’s experienced guidance, the department will offer a comprehensive range of services, including troubleshooting, maintenance, and repair, tailored specifically to Flow Sciences’ products. The goal is to enhance customer experience and ensure optimal performance of their containment systems, marking a significant step in the company’s ongoing efforts to prioritize customer satisfaction and product efficiency.
Introducing Our SAF T FLOW Fume Hood Builder
Flow Sciences, Inc., renowned for its cutting-edge containment solutions, proudly introduces the SAF T FLOW Fume Hood Builder – a revolutionary online tool designed to simplify the process of creating the ideal fume hood configuration. With this user-friendly platform, you can effortlessly customize your fume hood, selecting the type, width, and base that perfectly aligns with your needs. Witness your vision come to life in real-time as you explore the combination of products, all while enjoying a hassle-free experience.
Introducing the Containment Process Builder (CPB)
Flow Sciences, Inc., a leading provider of containment systems for laboratory, pilot plant, and manufacturing has launched the Containment Process Builder (CPB),an online visualization and documentation tool that allows the user to build their very own containment process and visualize the combination of products in real-time.
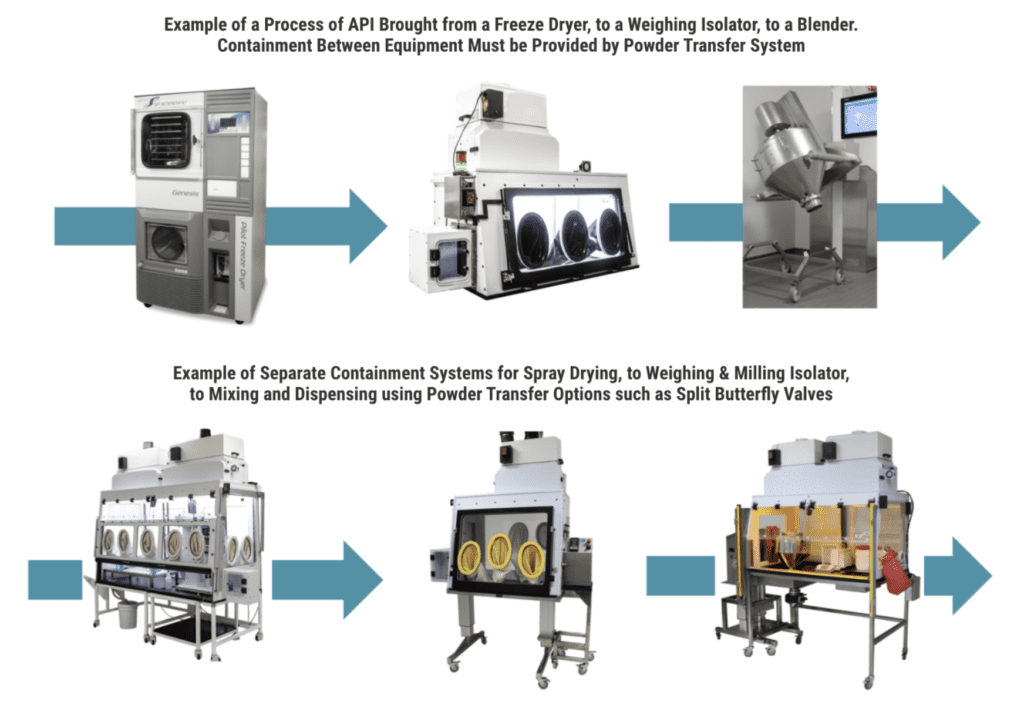
POWDER TRANSFER OPTIONS
In pharmaceutical manufacturing, applications that use a continuous process allow for transfer through containment. It is important for Flow Sciences to understand during the engineering …
Containment Engineering Controls for Cytotoxic HPAPI ADC
STERILE & NON-STERILE GLOVE BOXES AND CONTAINMENT SYSTEMS FOR HPAPI PROCESSING • WEIGHING / HPAPI SYNTHESIS • CONJUGATION & PURIFICATION • LYOPHILIZATION AND FILLING • …