Abstract:
In a previous White Paper 2, we reviewed in detail how Flow Sciences vented balance enclosures can allow accurate measurement of samples in the range of 0.1 mg to 0.1 µg.
In this paper, we will review why very bad things happen when either quantity or purity of Highly Potent Active Pharmaceuticals is not properly maintained during the compounding process. Additionally, when highly potent active pharma ingredients are not effectively contained, workers may be adversely affected.
Why Compounding Applications Require Superior Containment

In the last fifteen years, the number of compounding labs has dramatically increased in the United States. Because there is an increasing demand for more high potency products and the number of different products being compounded in each facility is growing, there is an increasing need for quality control worldwide in these labs.
Dramatic situations have occurred worldwide since 2002. Consider Table 1 below:
| Table 1: Issues With Contaminated Health Products | ||||||
| # | Name | Location? (Mfr.?) | Date | Impact | Cause | Footnote |
| 1 | Cefotaxime | Germany | 2002 | Not reported | Particluate Matter in Injectable | 3 |
| 2 | Cough Syrup | Panama (Chinese Mfg) | 2006 | 138 Killed | Diethylene Glycol impurity | 4 |
| 3 | Teething syrup | Nigeria | 2009 | 84 child deaths | Diethylene Glycol impurity | 5 |
| 4 | FDA Report (1990-2009) | US | 2009 | 34% of Compounded Drugs Failed Purity Tests | Drug potency, impurities | 6 |
| 5 | Chemotherapy Drugs | US NIOSH Lab Worker Study | 2010 | ~50% More Mutations than Control Grp. | Inadequate, improper containment equipment | 7 |
| 6 | Injectable epidural Steroid | US (New England Compounding) | 2012 | 753 Meningitis Cases; 64 dead | Contaminated Injectable Drugs | 8 |
| 7 | Sterile Meds (Injectables) | US, Texas (Specialty Compounding) | 2013 | 17 Rhodococcus Equi infections; 2 died | No GMP, many products, contaminated | 9 |
| 8 | Sterile Meds (Injectables) | US, Texas; ( IV Specialty) | 2015 | Unknown, unproven | Bad Containment, sanitation, and air flow | 10 |
| 9 | Marijuana, Medical Use | US | 2017 | 20 defective doses; 3 Deaths | Marijuana had fungus spores | 11 |
From insulin to various heart and cancer medications, highly accurate measurement is required during formulation of compound drugs. If compound purity and worker exposure issues are not resolved, modern compound pharmaceutical companies have the capacity to significantly harm both the patients and workers inside these labs.
Figuratively speaking, “red and green lights” in this process must be devised and obeyed. The author believes the following issues (red lights) with compounding equipment need attention (green lights):

Containment failure caused by poor internal airflow:
High potency powders must be contained within a designated space while being weighed or mixed with other ingredients. Active pharmaceuticals should never be in an environment where they can inadvertently spill or blow into the lab environment during the weighing or compounding procedures. Sometimes equipment design issues cause powder and fume containment to be compromised.
Also, compounding labs need to protect their constituent ingredients and blending processes from cross-contamination during processing. Escaped airborne trace pharmaceuticals are a significant contamination issue. If process protections break down, scenarios 4,5,6,7, and 8 from Table 1 could easily occur.

Solution:
Smoother air flow always minimizes the effect of turbulence outside of the enclosure, working to stabilize interior containment. The Flow Sciences Class I BSC (biological safety cabinet) achieves low turbulence by using four (rather than one) airfoils surrounding the rectangular face opening paired with a slotted rear plenum.
The resultant aerodynamics creates proven particulate and vapor containment using ASHRAE 110-2016 and ISPE approved surrogate powder containment protocols. ASHRAE 110 containment is routinely found to be 0.05 PPM or better; surrogate powder testing results are routinely at or below 10 nanograms per cubic meter.


Reduced weighing accuracy caused by fan vibration transmitted to the work surface:
It is crucial in analytical and compounding environments that precise weighing takes place inside the containment area. In modern measurement scenarios, lab balances are required to be accurate and reproducible with deviations ranging from +0.1 mg (milligrams) to +0.1µg (micrograms). Fan vibration in many units creates drifting tare weights. With unreliable weighing taking place, scenarios similar to 4 from Table 1 could occur.
Balance containment units from several manufacturers mount the fan below the filter housing and attach it directly to the containment cavity with sheet metal screws. This produces a direct contact pathway for motor vibration to be transmitted into the weighing area. Frequently in these units, fans must be turned off to get a stable balance reading.
Not good, particularly if the fan is located just above the work area where particles from the fan may contaminate the work area.

Solution:
Weighing stability is accomplished using the features highlighted below:
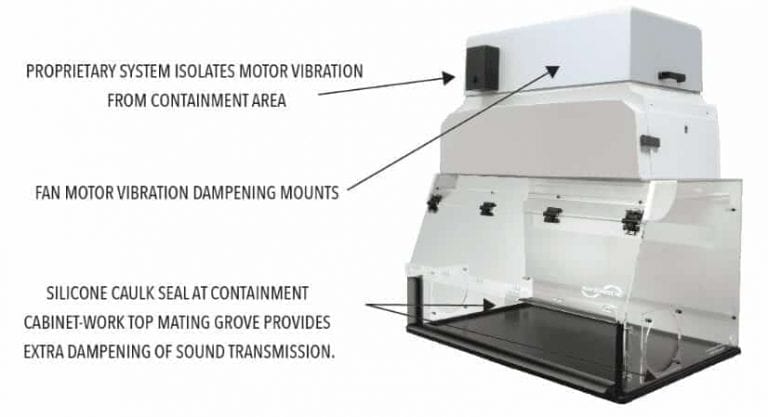
Little to no vibration in operating FSI balance enclosures means balance stability is achieved while the fan-driven containment system is running.


Functionality of equipment can be impaired by bad design:
Any containment system should support, not impede, effective science. If containment equipment cannot do this, precision is lost. All nine scenarios shown in Table 1 could occur with containment equipment of inferior design.
The Flow Sciences balance enclosures not only contain and protect Highly Active Pharmaceutical ingredients, they facilitate more straightforward compounding and sampling four different ways:

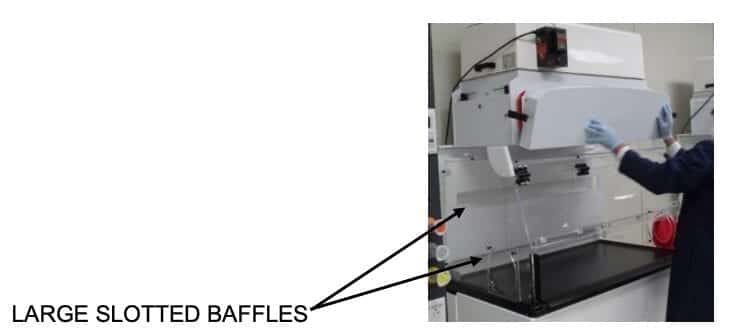
Ease of Cleaning assured with large slotted baffles:
Many balance enclosures have small holes or slots in their baffle assemblies which are very hard to clean. The sharp edges can cut skin or a glove and increase the chance for contamination. Flow Sciences uses straightforward large slotted baffles which can easily be cleaned.

Bag-In/Bag-Out filters can be replaced without threatening lab area contamination.
Many less expensive balance enclosures do not offer this option. An exposed filter is difficult to remove from a lab area without room contamination. Such contamination will create health issues and potentially contaminate other samples in the lab, destroying traceability. Photos show how bag-out process protects the room environment during filter change-outs.


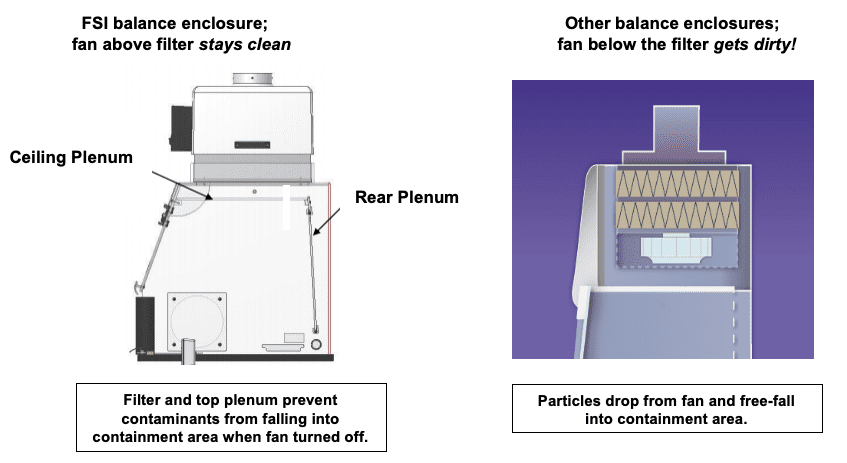
Filter system configured to stop back-contamination when the fan is switched off.
Some less expensive balance enclosures actually place the fan below the filter. This creates an area of positive pressure inside the fan housing and causes loose particles to fall down onto the surface inside the containment area when the fan is switched on or off. The Flow Sciences enclosure places the fan above the filter, keeping it and the fan housing completely clean of particles deposited on the fan blades trapped by the filter!

Thicker Filters mean longer life!
Flow Sciences balance enclosure filters are 4” thick to assure long life and infrequent motor RPM adjustments. Other manufacturers use thinner (2-3”) filters, which need to be replaced more frequently. Also fan adjustments must be made more often, and the fans usually produce more noise working against a clogging filter.


Conclusion:
All the above features allow Flow Sciences containment units to effectively contain fumes and powders, prevent cross-contamination, and be in compliance with the US Centers for Disease Control and Prevention Criteria for a Class 1 BSC (biological safety cabinet) with proven particulate and vapor containment.
When the safety of compounding workers and the general public are both at stake, no lower standard is acceptable!
Footnotes:
- Not valid in Ireland
- Critical Considerations when Selecting a Vented Balance Enclosure, Haugen, 2018, p2
- https://www.atsjournals.org/doi/abs/10.1164/ajrccm.165.4.2108033
- https://www.abc.net.au/news/2016-07-30/five-jailed-in-panama-over-toxic-medicom-cough-syrup-scandal/7674760
- https://www.safemedicines.org/nigerian-children-killed-by-contaminated-teething-medicine
- https://www.cdc.gov/niosh/updates/upd-09-01-15.html
- http://theoncologypharmacist.com/top-issues/2013-issues/may-2013-vol-6-no-2/15787-chemotherapy-and-pharmacy-a-toxic-mix
- https://en.wikipedia.org/wiki/New_England_Compounding_Center_meningitis_outbreak
- https://www.medscape.com/viewarticle/809315
- https://www.fda.gov/ICECI/EnforcementActions/WarningLetters/ucm449675.htm
- https://www.sacbee.com/news/local/health-and-medicine/article131391629.html

