Robert K. Haugen, Ph.D.
Director of Product and Technology Development
Flow Sciences, Inc.
ABSTRACT:
The galaxy of laboratory containment devices is incredibly diverse.
While many segments of this industry have developed their own categories of classification 1, no overarching system of categories has ever been developed for the entire lab containment equipment industry.
We offer such a model here and invite others to reflect upon it and offer comments on any refinements you deem necessary. The most powerful enemies of any model are time and discovery.
Nevertheless, here is our 2019 attempt to conquer the galaxy!
ESTABLISHED AREAS OF CONTAINMENT TECHNOLOGY:
We believe most lab containment products can be fit into one of these five categories: 1) Chemical Fume Hoods, 2) Powder / Pharmaceutical Containment Devices, 3) Biological Safety Cabinets, 4) Special Purpose Glove Boxes, and 5) Local spot ventilation.
Remember, these five areas are broad categories. Depending upon the given area, many sub-categories are likely. We examine each category closely below:
- Chemical Fume Hoods:
These units are almost always permanently installed in a certain location inside the laboratory. Their main purpose is the containment of vapors created during chemical reactions. Exhaust is typically carried out of the building and into the air via an exhaust stack. In limited circumstances, this exhaust may be filtered and subsequently exhausted or sent back into the laboratory.
A chemical fume hood is defined in ASHRAE 110-2016 2 as “a box-like structure enclosing a source of potential air contamination, with one open or partially open side, into which air is moved for the purpose of containing and exhausting air contaminants. A laboratory hood is generally used for bench-scale laboratory operations but does not necessarily involve the use of a bench or table.”
a) Counter top exhaust hoods:
These hoods are the most typical chemical exhaust unit and have many applications. They are almost always permanently installed in one location.
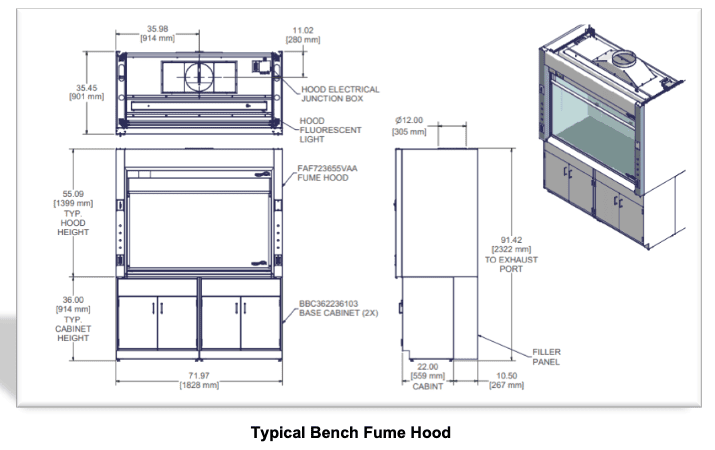
b) Floor-mount & purpose-built large exhaust units
These large devices usually serve as containment housings for large machinery and processes. These containment devices are so large that their exhaust requirements far exceed standard fume hood specifications. Such units may have filtered input air and exhaust and may be designed for production level distillation/refluxing, portable virulent disease treatment, or kilo-scale applications.
The writer presents the following as examples of Large Equipment Enclosures:



2. Powder, Pharmaceutical Containment Devices:
The devices in this category are primarily used in the formulation and analysis of dry, finely divided pharmaceutical powders. Because such applications under the wrong conditions can easily create a dangerous environment for process workers and scientists, surrogate powder testing in areas within 25 feet of such containment structures are typically specified at or below 10 nanograms per cubic meter.
Designing such a pharmaceutical containment device with unfiltered exhaust air leaving the building through exhaust stack is virtually never done.

These pharmaceutical containment devices come in a wide variety of sizes, filter options, and materials of construction. Flow Sciences estimates over 6,000 “standard” products are available considering these options alone!

As noted in an earlier article10,this widely divergent collection of containment units may also be linked together into process arrays to keep HAPI’s isolated during research or production procedures.

3. Biological Safety Cabinets:
Biosafety cabinets are defined as “an enclosed, ventilated laboratory workspace for safely working with materials contaminated with (or potentially contaminated with) pathogens requiring containment.” Several different types of BSC’s exist, differentiated by the degree of biocontainment required.4 Most biosafety cabinets send exhaust through HEPA particle filters and return this air to the laboratory or exhaust it outside. Without getting too far into the weeds, three classes of BSC’s exist:
a) Class I:
These cabinets provide personnel and environmental protection but no product protection. In fact, the inward flow of air can contribute to contamination of samples. Inward airflow is maintained at a minimum velocity of 75 ft. / min (0.38 m/s). These BSCs are commonly used to enclose specific equipment (e.g. centrifuges) or procedures (e.g. aerating cultures) that potentially generate aerosols. BSCs of this class are either ducted (connected to the building exhaust system) or unducted (recirculating filtered exhaust back into the laboratory).
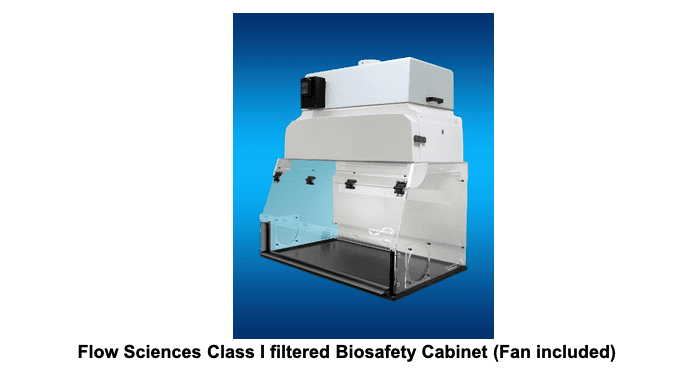
b) Class II:
Class II cabinets are the most commonly used in clinical and research laboratories. These cabinets provide personnel and product protection since makeup air is HEPA-filtered. There are five types of these units: Type A1, Type A2, Type B1, Type B2 and Type C1. Each type’s requirements are defined by NSF International Standard 49. About 90% of all biosafety cabinets installed are type A2 cabinets.
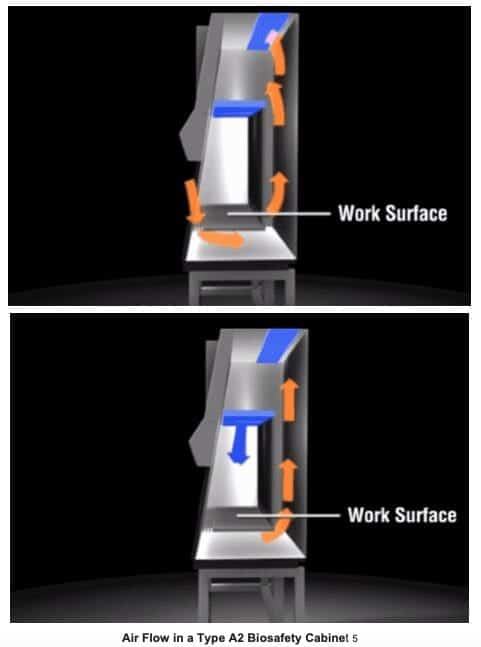
Principles of operation use motor driven blowers (fans) mounted in the cabinet to draw directional mass airflow around a user and into the lower interior front air grille – protecting the operator. The air is then drawn underneath the work surface and back to the rear vertical baffle, then up to the top of the cabinet where it passes through the high efficiency particulate air (HEPA) filters. A column of HEPA-filtered air is then blown downward, over products and processes to prevent contamination. Air is also exhausted through a HEPA filter, and depending on the Type of Class II BSC, the air is either recirculated back into the laboratory or pulled by an exhaust fan through duct work and expelled from the building.
c) Class III:
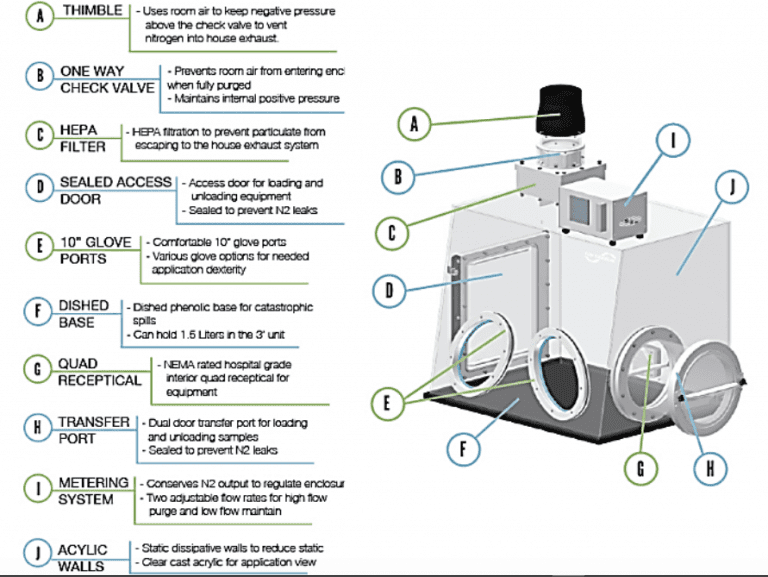
The Class III cabinet, sometimes called an isolator, is generally only installed in settings where contamination is particularly undesirable. It is specifically designed for work requiring USP requirements for sterility under chapter 1211. System shown below uses an H2 O2 generator for decontamination. Gloves attached to the front prevent direct contact with hazardous materials.

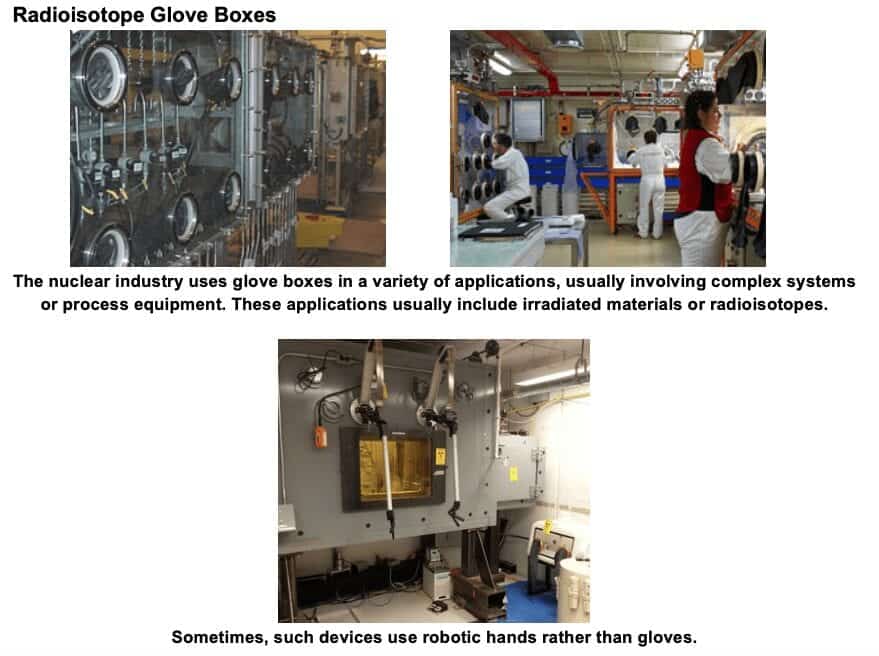
4) Special Purpose Glove Boxes
Glove boxes are also used in many divergent scientific areas not associated with the tightly-defined discipline of Biosafety and NSF 49.
a) Hybrid Glove Boxes
Flexible hybrid units which add minimal air flow and removable front panels to the Biosafety model have been used in several different pharmaceutical applications involving High Potency Active Pharmaceutical Ingredients (HPAPI’s), including compounding and sampling.


b) Specialized atmospheric glove boxes with very limited exhaust
Many chemical and radiological applications require either very dry or oxygen-free conditions. This condition can be achieved with either a pure nitrogen or inert gas atmosphere. Such applications include pyrophoric substances, hygroscopic materials, and radioactive metals that oxidize aggressively. As a consequence of such a sealed environment, containment is also achieved.
c. Integrated Glovebox arrays for HPAPI Processing

With HPAPI (High Potency Active Pharmaceutical Ingredients) or API materials, it is frequently advantageous to link process-specific modules together so an entire process of formulization can take place inside an isolated, multi-step environment. The number and types of such modules that can be linked together is virtually without limit.
5) Local Exhaust Ventilation:
Several types of localized ventilation serve the research needs of the 21st Century. The functional caveat of these technologies is that they are an inexpensive technique for managing less toxic fumes or heat using an exhaust source in an area not confined by walls or a sash. As such, their “containment” effectiveness is very limited.
These units show effective localized removal of heat and non-toxic odors with reduced exhaust requirements compared to chemical fume hoods. Canopy hoods and funnel exhaust systems are examples of this containment type.
a) Canopy Hoods:
Canopy hoods are effective with high temperature fumes in close proximity to the canopy. They are far less effective if fumes are emitted at room temperature or more than 18” vertically away from the canopy.

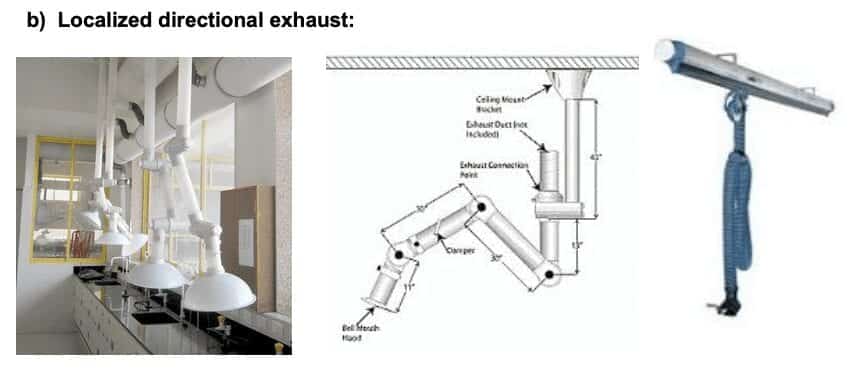
These systems are most effective with very small quantities of fumes where the exhaust port can be maneuvered very close to the fume emission point. If these conditions are not met, these devices will not be effective.
Another Model Reveals Similar Containment Groups!
While I was working on this paper, Ray Ryan and myself were also trying to sort containment groups by function and protection.This was being carried out to support the expanding need for SEFA standards (SEFA is Scientific Apparatus and Furniture Association) for newly emerging products.
Here is an array of products defined by whether devices protect personnel, product, or both.
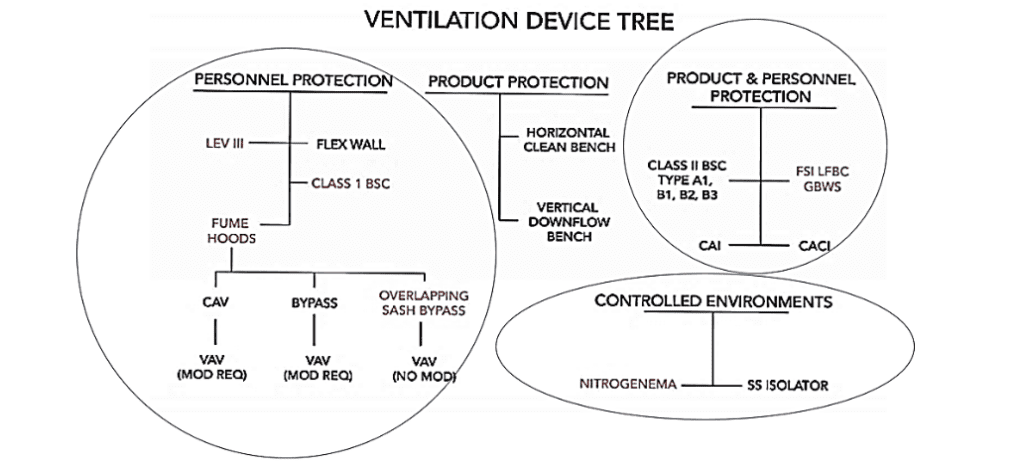
Over this model, I have circled the categories where Flow Sciences has products. Again, we are covered in three of the four product categories identified, only missing devices that protect products, but not personnel. This diagram confirms our company’s commitment to protect personnel, while offering us the opportunity to develop new products which keep products clean while not protecting personnel (uncircled tree). Many electronic component manufacturing fields emphasize this uncircled category.
Summing It Up:
There is a huge variety of laboratory containment devices. We can group these devices into either five or four categories depending on which model of classification we use. One model sets forth types of commonly grouped containment devices. The other model classifies containment devices that protect product, personnel, or both.
Containment groups are summarized below first by established groups, and second by product/personnel protection characteristics.
| Cat # | Cat. Name | Product Markets | Containment Level | FSI Products | |||
| Ed. & Gen Chem. | Pharma | Nuclear | Bio | ||||
| Established Areas Model | |||||||
| 1 | Chem. Fume Hoods | X | X | X | X | < 0.05 PPM 1 | Y |
| 2 | Powder containment | X | X | <0.05 PPM 1 | Y | ||
| 3 | Biosaf. Cab | X | X | X | NSF Listed 2 | Y,(Cl.1 only) | |
| 4 | Special Purp Glv. Boxes | X | X | X | < 0.05 PPM 1, 5 | Y | |
| 5 | Local non contained ventilation | X | Various 4, 5 | Y | |||
| What’s Protected Model | |||||||
| 1 | Personnel Protection | X | X | X | X | < 0.05 PPM 1 | Y |
| 2 | Product Protection (6) | (Ag. & Chip) | Iso Class 5 7 | N | |||
| 3 | Personnel & Product Protection | X | X | X | < 0.05 PPM 1, ISO Class 5 | Y | |
- Defined by ASHRAE 110 2016; control Level set in AIHA Z 9.5
- Pass all tests NFF 49 or equivalent
- For localized canopy and funnel exhausters, there are no US standards for containment
- ASHRAE 110-2016
- Surrogate powder testing also frequently done with different levels of acceptance
- Since this class of product blows air outward into the lab, it does not protect the worker but does bathe the product in HEPA filtered air, keeping it free of contaminants.
- Iso Class 5, an ISO class 5cleanroom is designed to allow no more than 3,520 particles equal to or larger than 0.5 microns per cubic meter of air. This equates to a Class 100 cleanroom under the Federal Standard 209E, which allows for 100 particles (0.5 microns or larger) per cubic foot of air.
Any company in search of a mission statement or identity may find the “FSI Products” column in the chart illustrative. It will show areas of competence and involvementin the containment. Conversely, it highlights areas of non-involvement which may also be new market opportunities.
Remarkable! A search to develop a market model has led to a way to define mission and highlight growth opportunities. This galaxy gives back!
Footnotes:
- Biosafety cabinets, for instance
- ANSI / ASHRAE 110-2016, American Society of heating, refrigeration, and Air Conditioning Engineers, p.4
- Flow Sciences, Haugen, Critical Considerations when Selecting a Vented Balance enclosure, 2018, P. 2
- https://en.wikipedia.org/wiki/Biosafety_cabinet
- The Eagleson Institute https://www.eagleson.org/products/productdetails/?id=31
- http://fumehoodstore.blogspot.com/2014/01/largest-fume-hood-genie-scientific-has.html
- Installed isolation area in pharmaceutical lab (Flow Sciences)
- When connecting a FS Fan/Filter to house exhaust, Flow Sciences recommends the use of a Thimble Connection to provide safe exhausting of the Fan/Filter Housing. Since each Fan/Filter Housing has a built-in fan downstream from the filter, the connection should be made with the Flow Sciences’ Thimble Connection which has been designed to be slightly larger (5″ID) than the discharge duct from the Fan/Filter Housing which is 4″ OD. The thimble is then fitted with 4″ID flex hose which runs to the general exhaust. The house exhaust should pull more CFM that the Fan/Filter is exhausting. For example, if the Fan/Filter Housing is exhausting 100 CFM, Flow Sciences recommends the General Exhaust be set to 115 CFM. The purpose of this is to prevent fluctuations in the general exhaust from adversely affecting the performance of the enclosure.
- flowsciences.com
Designing and Testing Containment Systems for High Potency Active Pharmaceutical Ingredients, Dr. Robert K. Haugen, Director of Product and Technology Development), 2018, Flow Sciences Inc.; https://flowsciences.com/designing-testing-containment-devices-used-high-potency-active-pharmaceutical-ingredients/

