POWDER TRANSFER OPTIONS
In pharmaceutical manufacturing, applications that use a continuous process allow for transfer through containment.
It is important for Flow Sciences to understand during the engineering and planning phase where the hazardous material is coming from and how it is entering the containment system, as well as how it is exiting and where it is going.
Localized Containment for Process Equipment
Providing Containment for the hazardous area rather than using a large cleanroom or respirator program allows for more consistent and higher performance containment with lower energy and operating costs. In many applications, this allows for weighing or dispensing to happen in the same unit, saving valuable lab space and transfer time.



Containment System to Containment System
Transfer Option into Containment System

Containment System to Transfer Option
Containment System to Waste Disposal

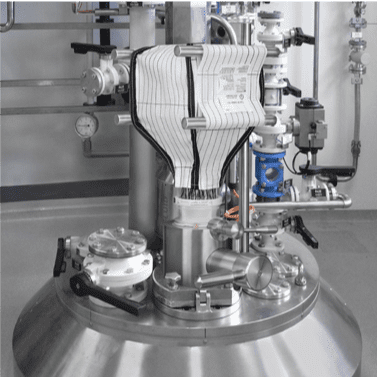
4” Sanitary Fitting through the Base of the Containment System
8” Sanitary Fitting through the Sidewall
of the Containment System
Both 4” and 8” Sanitary Fittings
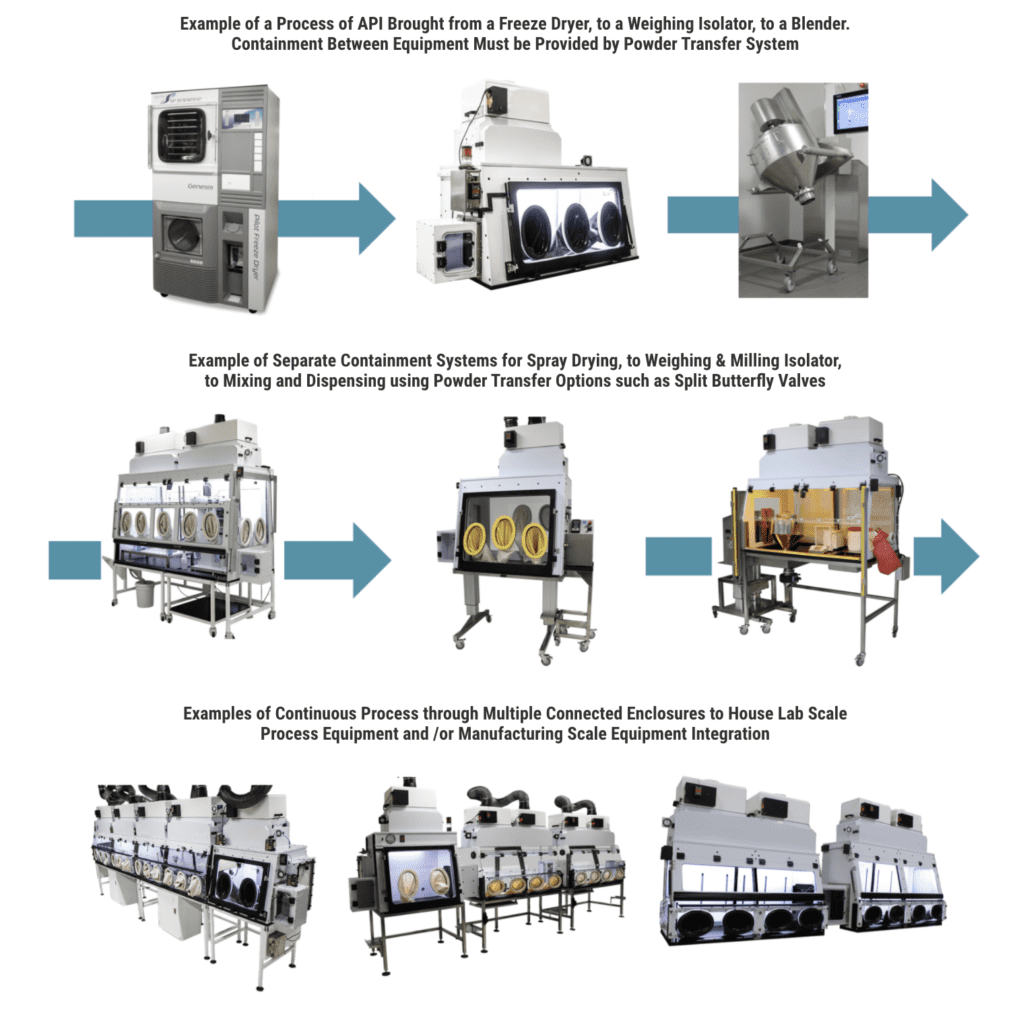
FSI’s continuous liner is an 8 or 12-inch stainless steel port designed for bottles, IBCs, or large quantities of waste disposal while keeping an ongoing process under containment.
This eliminates cross contamination of powders, protecting the personnel as well as the product. The continuous liner kit functions much like a bag-in/bag-out filter change-out, in that waste or used materials are placed through the port into a 25’ bag, tied and taped off, then cut free and removed from the enclosure.
Using correct procedure, the next section of liner will be clean and ready for use during the next cycle.



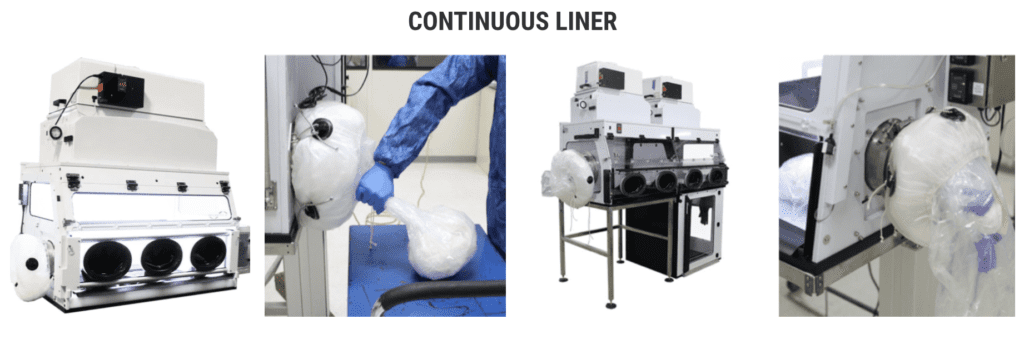
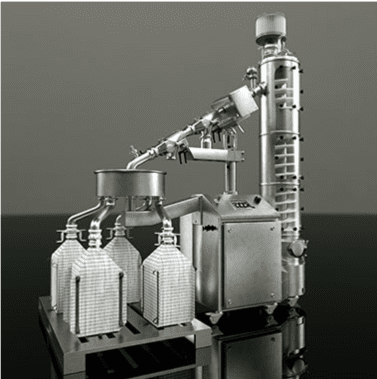
The manufacture of pharmaceuticals in powder or tablet form often involves handling toxic or highly sensitive solids that can pose a direct threat to people’s safety. Cross-contamination with other raw materials and active ingredients likewise has to be ruled out at all times. To deal with these and many other challenges related to decanting and filling highly sensitive bulk goods, Rommelag developed the Flecotec system – a brilliant containment concept that can be ideally adapted to existing processes, requires no new certification, and reduces the subsequent cleaning work to a minimum.
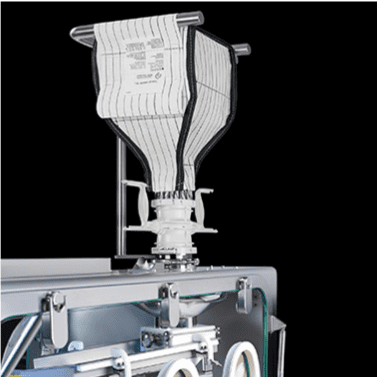
EMPTYING A BAG
Part of the Flecotec system is an innovative emptying procedure, Flecotec MTS (Material Transfer System), where Flecotec closures are used as a secondary interface, as in bag filling.
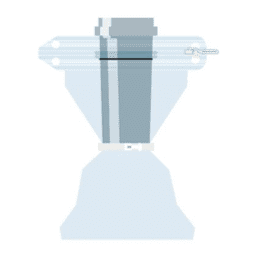
FILLING A BAG
Perfect for swift and secure connections – the Flecozip bags with various connection components allow for fast material transfer.
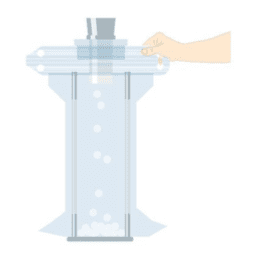
FILLING TABLET BAGS
The Flecotec system also includes specially developed bags and fasteners for the filling of tablets.

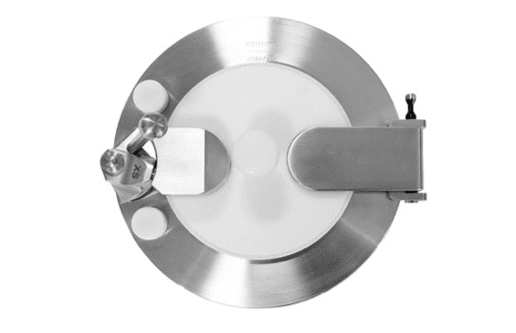


DPTE® Alpha
The core of the DPTE® transfer system is the Alpha port with its secure interlock enabling totally safe connections and disconnections.
The DPTE® system enables material to be moved from one sterile zone to another through a non-sterile zone, with leak-tight, risk-free reconnection.




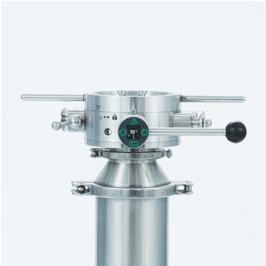
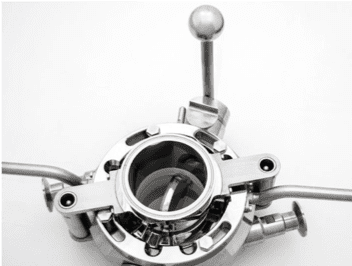

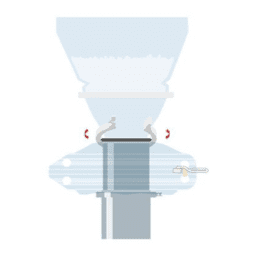
PharmaSafe® valve offers numerous benefits including the reduced risk of contamination, meeting GMP and product quality requirements, maximizing yield by transferring poorly flowing and high value products and removing costly secondary barrier containment and cumbersome PPE.
- ChargePoint PharmaSafe® – containment down to 1µg/m3
High containment performance with no additional seals, vacuum or extraction required.
- ChargePoint PharmaSafe® pro – containment down to 0.1µg/m3
High containment performance with a simple, low volume extraction
- ChargePoint PharmaSafe® excel – containment <0.1 µg/m3
Advanced performance to nanogram levels in a compact efficient split valve.



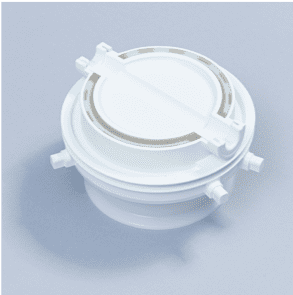
Single Use Split Butterfly Valve Passive
The ChargePoint Technology SUP (Single Use Passive) is a contained and economic solution for the transfer of powder ingredients between process steps or even facilities.
The ChargePoint® SUP is a contained powder transfer interface for pharmaceutical manufacturers that need to economically transfer powder ingredients between process steps or even facilities.
SUPs function as the passive mating half of the ChargePoint Split Butterfly Valve system, delivering outstanding containment and sterility assurance performance whilst eliminating the time and cost associated with cleaning, maintenance and validation


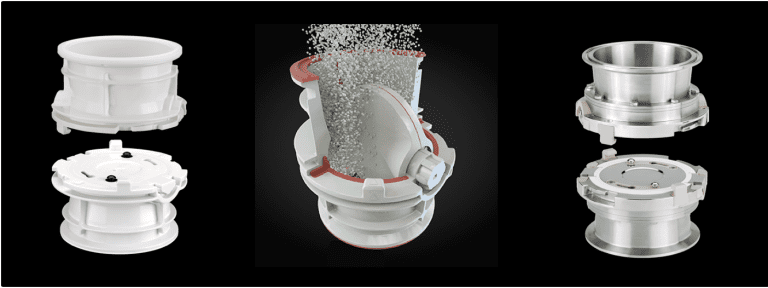
AVAX is the trend-setting and unrivalled split butterfly valve technology from Andocksysteme. Completely made of medical-grade plastic materials it represents the origin for a new generation of single-use containment interfaces.
Strictly designed as a congruent passive to passive system, this unique valve enables its usability in any desirable application due to its interoperability.
- Meets OEB 5 requirements (STTW ≤ 1 mcg/m³)
- Intuitive and secure handling
- Tri-Clamp seal integrated (4” BS 4825-3)
- Hybrid operation with stainless steel AVAX optional
- Sterile execution available



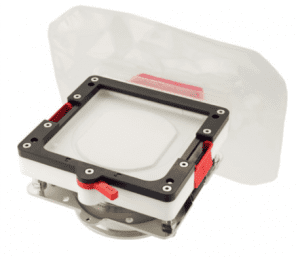
Ezi-Flow™ CSV4 & CSV6
The cornerstone of the Ezi-Dock™ High Containment solution is the Ezi-Flow™ CSV Transfer System. Available in Multi-use and Single use formats and 4 and 6 inch bores, the Ezi-Flow CSV breaks the mould, providing superb levels of containment at greatly reduced cost.

Ezi-Flow™ CSV4 All Plastic
Ezi-Flow™ CSV All Plastic, Single-Use High Containment System provides a highly effective but simple and disposable transfer solution for the Pharma and Biopharma industries. Innovative design and construction provides the ultimate combination of high containment and low cost.



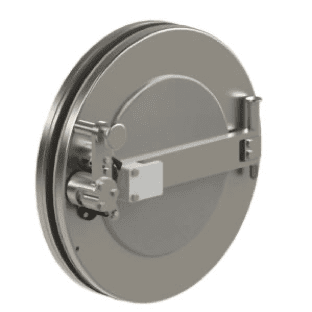
Rapid, repeatable transfer without breaking containment.
The CRL Rapid Transfer Port (RTP) is a Double Door Transfer System utilizing our proven transfer technology. This system consists of two main components, the alpha flange and the beta assembly. The Alpha flange is normally mounted to the wall of a glovebox and the Beta assembly is mobile and connected to a container, bag, or process component. When the two assemblies are mated, hazardous materials can be rapidly transferred in and out of the glovebox without breaking containment, while maintaining the safety of the operators.
Available standard sizes (mm): 105 | 190 | 270 | 350 | 460

