
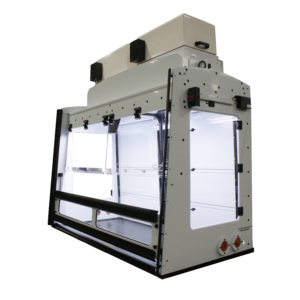
Analytical Pill Press and Hardness Tester Sliding Sash Enclosure
This sliding sash hood enclosure is designed for personnel protection during applications involving powder hazards. It is engineered to contain processes involving a Natoli Tablet

Nitrogen Purge Enclosure for Tablet Pressing, Tablet Coating & Tablet Drying
This custom-built Nitrogen Purge Enclosure is a custom-built, multi-process containment unit designed to provide personnel and product protection. It creates a low relative humidity environment

Stainless Steel Glovebox for Cytotoxic Drug Development
Stainlees Steel Glovebox for Cytotoxic Drug Development designed to provide personnel protection while working with powder substances. The enclosure features include 316 stainless steel superstructure, hinged

Lindberg/Blue M Vacuum Oven
DESCRIPTION For containing vacuum oven where pressure and heat must be controlled for drying, curing, vacuum embedding, and plating applications. Enclosure features an aluminum frame,

Particle Analysis Suite
Professionals in the field of laboratory sciences occasionally encounter a situation where they must enclose several processes requiring the manipulation of product in two or

