ABSTRACT:
- A fire breaks out in your fume hood.
- What do you do?
- Should you put it out? Should you run? Should you turn off the hood fan?
- Should you activate the building fire alarm?
- Wait a minute! Are YOU on fire????
Very little exists in one place to answer these questions. Policies actually differ. Accountabilities are sometimes unclear. This White Paper examines all these issues in as clear and direct way as possible. One thing is clear: these questions need to be thoughtfully addressed before the fire starts!
The Historical Perspective:
UCLA had a tragic safety episode in 2008. A fume hood lab fire killed Sheri Sanji, a research assistant. Any lab safety incident, including a fire, is likely preventable. Fire is a very publicly visible symptom of poor lab safety. It is up to those engaging in research to assume front-line responsibility for their own safety. Fire is one kind of accident that occurs in fume hoods; many other misadventures are also possible inside such a containment area.
Michael Wright1 correctly analyzes the proper safety perspective for such tragedies: “Our own (safety) investigations are about causation and prevention, not guilt. We believe in accountability, and we support civil and criminal penalties where they are appropriate – as I would argue they are here (UCLA). But that’s not our goal. The real issue is how we prevent such tragedies in the future. And from what I have seen, academic labs have a ways (sic.) to go. I don’t know any industrial lab director who would claim that he or she is not responsible for safety. PI’s (Principal Investigators) are equally responsible. We won’t make progress where they don’t acknowledge it.”

Unfortunately, many laboratories are not organized effectively to collect safety data, let alone improve adverse conditions.
Chemical fume hoods found in high school, junior college, and college chemistry labs are found to have many more accidents than hoods in commercial labs.2
One study by Dow, DuPont, and Corning cites an OSHA report concluding a college researcher is eleven times more likely to be hurt than a researcher in a commercial lab. Reasons pertain to cultural differences between how educational and commercial labs operate, safety priorities taking second place behind academic research goals, and countermeasures only being considered after something goes wrong.
John K. Borchardt confirms the differences between academic and commercial lab settings and their respective safety records on lab fires and accidents in 2013: 3
“Industrial and government labs generally have good safety records based on personnel training, safety inspections, and maintenance of equipment. However, the frequency of academic research laboratory accidents is more than ten times that in industrial labs.”
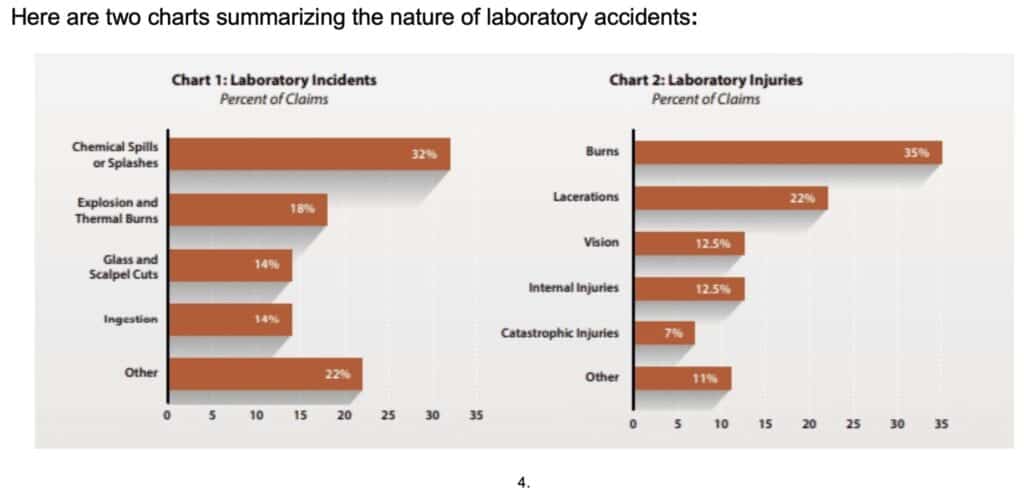
Chart 1 shows the types of lab accident incidents reported and the frequency of their occurrence. Explosions and thermal burns were the second most frequent type of incident, encompassing injuries caused by exposure to extreme heat such as from a burner or hot water.
Chart 2 shows the types of injuries resulting from these lab incidents. Burns and lacerations together accounted more than one-half of all reported injuries.
The dominant percentage of burns and lacerations in lab accidents are significant. Burns and lacerations are typical in fume hood fires. We can anecdotally fathom this situation from the ten examples cited in the chart below:
Ten Fume Hood Fires and Explosion Examples Located by Google:
| # | Date | Location | Casuelties | Cause | Remedies in Future | Web Address |
| 1 | 12/28/2008 | UCLA | 1 Dead | Improper Procedure, lack of Protective clothing, Uninformed Assistants | Drills, more visible equipment, train personnel | https://en.wikipedia.org/wiki/Sheri_Sangji_case |
| 2 | 1/7/2010 | Texas Tec. | 1 Serious injury | Making too much chemical, PI uninformed of SOP, removed explosive compound from hood to grind, BOOM | Use SOP, wear lab coat, wear gloves & lab coat & eye protection | https://www.depts.ttu.edu/research/integrity/CSB-response/downloads/TTU_EHS_accident_report.pdf |
| 3 | 10/27/2011 | Texas Tech | 0 Injuries | Explosion of acid waste storage bottles stored under fume hood | Do not allow acids and organic solvents to be mixed in same bottles | http://today.ttu.edu/posts/2011/10/statement-concerning-second-laboratory-accident |
| 4 | 11/13/2015 | UC Berkeley | 1 Injury | Explosion of a drypowder while being scraped from filter per SOP | Follow SOP and scrape while wet. Wear protective gear and double-glove, fix SOP. | https://ehs.berkeley.edu/lesson-learned-dry-scraping-causes-chemical-explosion |
| 5 | 3/16/2016 | U Hawaii, Manoa | 1 injury, arm amputation | Post Doc Investigator ignited 49 L tank with explosive gas mixture of O2, CO, and H2 with spark | 15 safety violations & $115K fine. See the article for details! Virtually all SOP’s ignored | https://cen.acs.org/articles/94/web/2016/09/University-Hawaii-fined-115500-lab.html |
| 6 | Spring 1997 | U Kentucky | 1 student minor injury | halogenated organicsolvents were involved, but the exact cause may never be known. | Do not mix solvent and nitric acid (see footnote 5) | |
| 7 | 9/29/2011 | U of Maryland | 2 Students 1st and 2nddegree burns | Waste acids mistakenly added to an organic reagent bottle. | Do not re-purpose reagent bottles as waste containers. Upgrade SOP. Have instructor review before experiment. | http://cenblog.org/the-safety-zone/2011/09/explosion-at-the-university-of-maryland/ |
| 8 | 4/26/07 | U Cal Irvine | 1 student, first degree burn and cut as he raised FH sash | Explosion of diethyl ether and toluene derivatives on a hot plate. | Tolunesulfonochloride SOP needed improvement. Temperature too high on hotplate. Find alternate for diethyl ether. | https://www.ehs.uci.edu/salerts/AlcoholTosylationExplosion.pdf |
| 9 | 3/2012 | Texas Tech U | 0 Injuries | Sulfur-Metal reaction in waste bin on floor outside fume hood | Properly segregate waste materials. Improve SOP’s. Consult with EH&S | https://www.depts.ttu.edu/research/integrity/lessons-learned/march-2012.php |
| 10 | 6/24/14 | Oak Ridge Nat. Lab | 0 Injuries; 6 evacuated | Spontaneous hotplate activation ignited a fire in hood while unattended. Another possibility is postulated below using a photograph of the incident in question | Unplug equipment when not in use. | https://opexshare.doe.gov/lesson.cfm/2014/6/24/4332/Deflagration-and-Fire-from-Malfunctioning-Lab-StirrerHot-plate/?responsive=false |

This information can be summarized as follows:
1. Fume hood fires are a significant problem mostly found in institutions of higher learning and academic research.
2. Based on post-accident analysis, reasons for accidents include:
- Little or no training of lab personnel on operating critical research and safety equipment
- Little knowledge of the properties of the chemicals involved
- Poorly formulated or non-existent SOP’s
- No peer (or supervisor) review of proposed procedures
- Little knowledge of required yields or separation procedures
- Poor or non-existent in-lab supervision of the student/researcher
- Poor monitoring and disposal of lab waste
- Poor lab attendance record-keeping which blurs accountability
- Failure to keep reacting materials inside the fume hood containment area
- Failure to properly remove discarded chemical waste from the fume hood or underlying base cabinets
- Failure to institute safety procedure changes after any given accident. Three of the ten accidents in the chart above all were similar and all occurred at the same institution within three calendar years.
3. Finally, we need to step back from any procedure before it is done and ask four questions:
- What, exactly, are we doing?
- How are we doing it? Can experiment be done in a short period, or will several staged sessions be required?
- What could go wrong?
- Where are safety resources?
- Extinguishers; which should be regularly inspected and assigned to the work area accordingly by fire class (e.g. A, B, C, D, etc.)
- Fire Blanket
- Drench shower
- Fire alarm
- Master power switch and gas shut-off
- The exit; should always remain unlocked and accessible
Going back to the questions posed in the abstract, none can be answered without having a safety plan similar to the one outlined above. 4
Answers to Abstract questions:
What do you do? Should you put it out? These questions are addressed in the bulleted list from the previous section. If a clear list of materials and an understandable procedure have been established, many difficulties will have already been defined and anticipated, including whether extinguishing the fire itself is a good idea.
Should you run? You should probably walk to some or all of the items in the list, depending on contingencies already considered in the planning stage of the experiment.
Should you turn off the hood fan? Fire and facility ducting practices in different areas of the USA affect the answer to this question. Some areas require hood exhaust fans be automatically disabled if duct smoke or heat is detected. In many other places, local codes require ducts be heat-isolated and fans remain operating during smoke and heat detection. Checking with mechanical personnel at your facility will help determine procedures to be used in a given facility.
Should you activate the building fire alarm? Know fire alarm activation policies, but in cases of an active fire in a lab, the answer to this question will most often be yes.
Wait a minute! Are YOU on fire???? Here, we really need to step backward a bit, ANY experiment with fire dangers present, should never be done solo. Using a fire blanket or other personal protection usually requires two people to be effectively carried out. The one death outlined above occurred with other individuals in the same lab working on other projects (e.g. UCLA, line one on table above). These individuals were not informed about the nature of the disaster-destined procedure and predictably participated ineffectively in the emergency.
Footnotes:
- Michael Wright, Director SHE, United Steelworkers 10/5/2018, American Chemical Society CHAS web conference
- Jon Rvans, Royal Society of Chemistry, Safety First?, 2014, https://www.chemistryworld.com/features/safety-first/7413.article
- Running Your Labs Like a Business, John Borchardt, 2008, Lab Manager Magazine, https://www.labmanager.com/business-management/2008/07/running-your-lab-like-a-business#.XBkYj1xKiM9
- SUPPLIMENTAL: EduRiskTM provides education-specific risk management resources to colleges and schools, and is a benefit of membership with United Educators (UE) Insurance, 2014, https://edurisksolutions.org/WorkArea/DownloasAsset.aspx?id=1894…135
- University of Kentucky Fire (Chart, Item 6)

