Abstract:
Pharmaceutical manufacturing is now undergoing significant transformation. COVID-19 and its pressure on nearly every aspect of human life has put a gigantic emphasis on rapid development of prescriptives, vaccines, and accelerated means for pharmaceutical mass-production.
Newer highly potent active pharmaceutical ingredients (HPAPI) hold great promise for the health of the planet, while posing manufacturing challenges for plant workers and their packaging and validating associates. As we design such facilities, we are likely to produce new equipment and containment strategies that look nothing like what we have today.
Flow Sciences’ role in this evolution must be to develop containment devices that complement the different and unique types of equipment we already know will be present. These changes must allow lightning fast research, valid conclusions, rapid development of production strategies, and increased facility efficiency and worker safety.
The writer will characterize the new production directions in pharmacy and describe flexible equipment to improve pharma efficiency, purity, and production safety in the coming decade.
Discussion:
The design of modern pharmaceutical facilities follows a series of factors during its development 2. Ahmed Salah Abu Shoukka frankly details this structure as shown below:
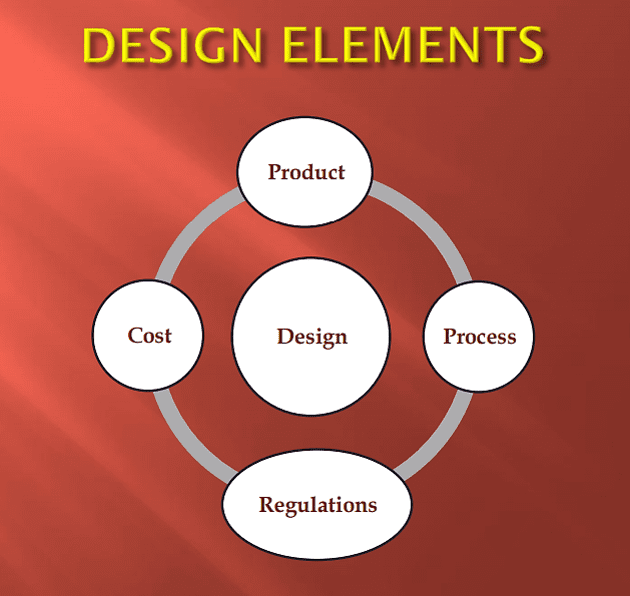
Shoukka includes four key components in his description of pharma manufacturing design: Product, Process, Regulations, and Cost. Based on the writer’s own experience, I have described below key elements of each of these four components.
- Product (pharma management, facilities planners, and production managers)
The product and demand for it in the pharma marketplace will determine the scale of production, size of the facility, and the type of machinery used in the drug’s manufacture. The most well-known of these new influences is COVID-19, whose pressure is so great on all our existing systems that production issues are being faced in tandem with developmental research. 6
- Process (biologists, chemists, biological and chemical engineers, plant design engineers, health & safety officers)
The biological and chemical processes used to manufacture the product will define the types of material containment required. As an artifact of this decision, batch or continuous manufacturing techniques will typically both be present in the production area.
It is always challenging for a manufacturer like Flow Sciences to predict what shape such containment will take. Flow Sciences must therefore maintain a diverse line of basic engineered options to house new production equipment compatible with manufacturing objectives.
FSI makes seven basic categories of equipment now:
Vented Balance Safety Enclosures
Local Exhaust Ventilation Hoods
Chemical Fume Hoods
Nitrogen Purge Gloveboxes
Compounding Hoods
Hybrid Isolators
Glovebox Workstations
In addition to these basic categories, we produce a significant number of larger transparent enclosures for process-specific applications.


- Regulations (compliance officers, environmental engineers, analytical scientists, and certification specialists)
The purity requirements and toxicity of each manufactured product will determine cross-contamination and worker protection standards for any containment apparatus. Communities close to the facility will insist air and water effluents and solid waste products released during manufacture are low enough to meet state and federal environmental standards.
Flow Sciences is used to these requirements. We frequently apply containment tests to measure and evaluate our containment devices 5. This equipment routinely passes less than 0.050 ppm of tracer gas under ASHREE 110 test conditions into the test environment, and surrogate powder releases are always between (1mg to 5ng) per cubic meter depending on the challenge presented by the application 4.
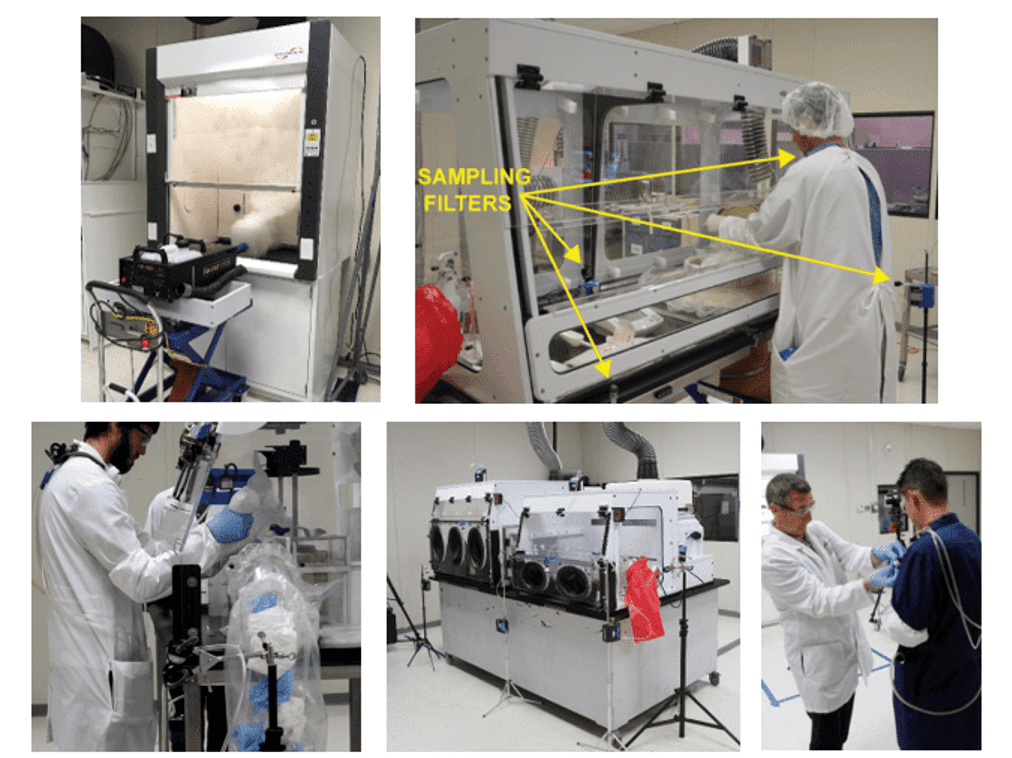
- Costs (production control, product flow, cost accounting)
Particularly with more established drugs, lean production is critical. This means efficient use of space and movement of intermediate bulk containers (IBC’s) from one temporary resting place to another. Such steps will also boost purity by minimizing cross-contamination of intermediate byproducts and precursor transfer.
The pandemic challenges we all now face will also put design pressure on companies supplying the pharma industry to control containment costs, which is an important factor in making new pharma products affordable worldwide.
As mentioned earlier, speed in addressing such needs is also crucial. This speed requirement has already produced commitments in principle to begin later stages in production acquisition while basic pharma is still being alpha and beta tested.6
ILLUSTRATION: How the Four Factors Cited Above interact with other!
The characteristics of oral solid dosage (OSD) manufacturing facilities can illustrate how these four factors interact. Lockwood3 recommends minimizing redundant labor costs caused by intermediate products moving from one batch to the next. Up until now, batch production appears to be the archenemy of production efficiency.
When designing a pharmaceutical OSD manufacturing facility, the ways in which materials move from one stage of production to another should be considered from the very beginning. We discuss how materials handling processes can, and should, influence building design for lean productivity.
-
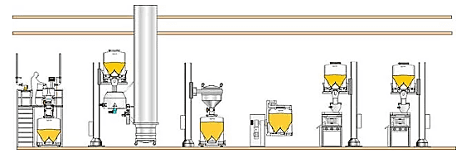
Fig. 3: Single Floor facility -
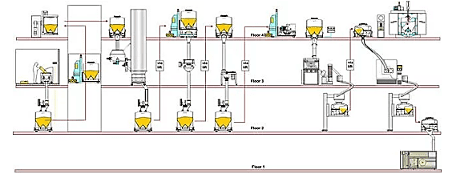
Fig. 4: Multiple floor facility
A single floor facility is the cheapest to build or lease, but ceiling height can impose constraints on materials handling solutions. However, even with small, single-floor buildings, intermediate bulk containers (IBCs) can be used to achieve full batch transfer from one process to another, thus reducing wasted product. Creative use of frameworks or mezzanines can enable the use of efficient gravity-fed vertical transfer systems, too.
Expensive, but with lots of available space, multiple-floor facilities are endlessly flexible. However, space can be wasted if due consideration is not given to the factory layout and overall production flow. IBCs can make the best possible use of height and space, while at the same time providing the opportunity to embrace new technologies to maximize the efficiency of each production area.
Several large corporations have founded subsidiaries that specialize in assisting pharma giants at layout efficiencies and production layout planning. Many will operate these facilities themselves in consort with the drug developer. Patheon (Thermo Fisher)7, Emerson Resources 8, and Recro 9 are all examples of businesses that have been commonly referred to as CDMO’s (Contract Development and Manufacturing Organizations)
Can Devices Used in Pharma Research / Manufacture be Successfully Housed?
Up to this point, we have reviewed factors that determine the process used in operating a pharma manufacturing facility. We have given an example showing how batch and continuous manufacturing can lead to effective results by considering the product, the processes, cost, and applicable regulations.
We have also suggested an ideal partner for providing separation, containment, and safety for such operations should be able to:
- Provide quick solutions for standard applications with a wide variety of standard product:
- Have larger housing strategies for accommodating continuous flow situations:
- Be prepared to combine existing technologies for more complicated processes:
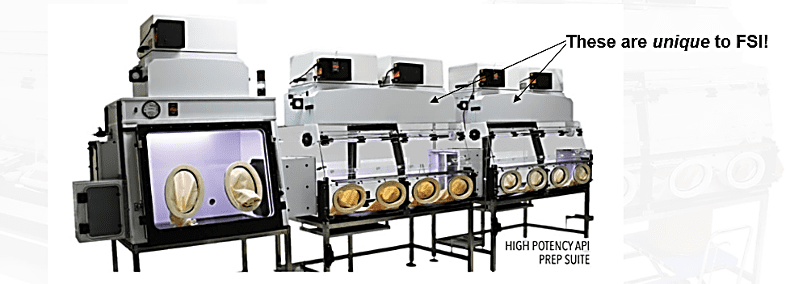
- Have engineering skills which enable specialized testing of such equipment:
Footnotes:
- Cell & Gene architecture services, Pharmaceutical Process Architecture Services, 2020,
https://www.cellandgene.com/doc/pharmaceutical-process-architecture-services-0001 - Design of Modern Pharmaceutical Facilities, Advanced Techniques in Biology and Medicine, Ahmed Salah Abu shoukka, Copad Pharma, 2017, frame 2,
https://www.longdom.org/open-access/design-of-modern-pharmaceutical-facilities-osd-2379-1764-1000202.pdf - The Ideal Design of Pharmaceutical Manufacturing Plant steps, 2017, Matcon Blog, Richard Lockwood, Pharma Business Line Director, Matcon Limited, England, https://www.matconibc.com/blog/your-what-kind-of-building-do-i-need-the-ideal-design-of-pharmaceutical-manufacturing-plant
- Five tests for Containment, Dr. Robert Haugen, Flow Sciences, 2020, https://flowsciences.com/five-tests-for-containment/
- ANSI-ASHRAE 110-2016, Human as Mannequin test, ANSI-AIHA Z 9.5, EN 14175, Good Practice Guide: Assessing Particulate Containment Performance of Pharmaceutical Equipment,
2nd Edition, 2012 - The $1 billion bet: Pharma giant and U.S. government team up in all-out coronavirus vaccine push By Jon Cohen, Science Magazine, Mar. 31, 2020 , 5:50 PM
We’re jointly investing in the R&D part of this, and that will bring us, hopefully, to approval. And then in parallel, we’re investing more in the manufacturing, so we are creating additional capacity. Of course, it’s step by step—it has to work—but there’s no hesitation now to do everything in parallel. When we have clinical data, we will have the capacity to scale up to very large quantities. That is the short and long story.
https://www.sciencemag.org/news/2020/03/1-billion-bet-pharma-giant-and-us-government-team-all-out-coronavirus-vaccine-push - https://patheon.com/logistics-services/
- https://emersonresources.com/?_vsrefdom=googleppc&gclid=EAIaIQobChMItNGn54XA6QIVxdSzCh1aUwLaEAAYAiAAEgJB4vD_BwE
- https://www.recrogainesville.com/?gclid=EAIaIQobChMItNGn54XA6QIVxdSzCh1aUwLaEAAYAyAAEgJEnvD_BwE