Abstract:
Methods and techniques used in pharmaceutical manufacturing are broadly reviewed. There are two basic models of pharma manufacturing: batch and continuous. Both approaches are defined. It is concluded that both approaches have elements of the other. Generally speaking, many pharma manufacturing procedures are transitioning from the batch to the continuous approach.
Based on this finding, it becomes self-evident that basic containment devices used in either manufacturing approach should be adaptable to the other.
Background:
Perhaps some of the most common pharma manufacturing techniques being used today are listed below 1:
1.) Solvent extraction:
2.) Milling

3.) Cooling
4.) Powder feeding

5.) Powder blending

6.) Granulation
7.) Encapsulating

These techniques plus other methods have been used in the batch production of pharmaceutical products 2. A generic example using these techniques might be:
Step 1:
Starting raw materials purified, then dried, using solvent extraction. (Batch 1)
Step 2:
Batch 1 then milled for uniform particle size. (Batch 2)
Step 3:
Batch 2 then cooled to uniform, sustained temperature. (Batch 3)
Step 4:
Batch 3 fed and
Step5:
Blended and mixed with other products (Batch 4)
Step 6:
Encapsulate batch 4 medication using hot melt extrusion or other technology (Batch 5)
The number of steps is different from the number of batches. This discrepancy occurs during steps 4&5 where many components are mixed together. This step gives us a big clue to an important possible route to simpler fabrication of pharmaceuticals.
A way that has fewer steps, fewer intermediate products which must be temporarily set aside or moved elsewhere, less human handling or warehousing of possibly hazardous intermediate products. This method is called Continuous Manufacturing. It has attracted much attention lately as being a faster, cheaper, and safer way of manufacturing pharmaceuticals.
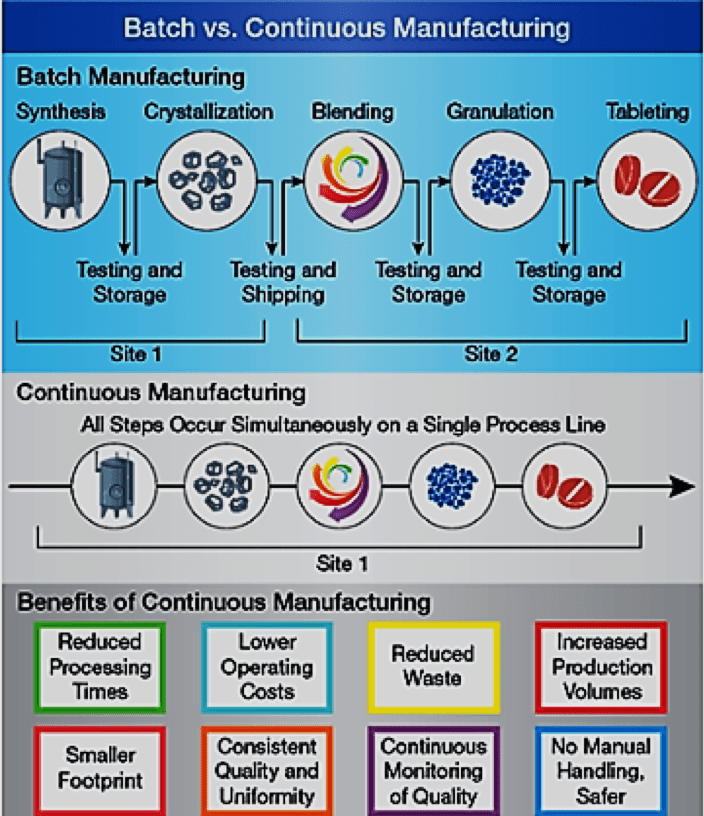
Many articles have been written about continuous manufacturing (CM). In a perfect CM world, there are no batches. Ingredients are added and processes undertaken with quantities produced by the previous step. The following diagram compares batch and continuous manufacturing 2:
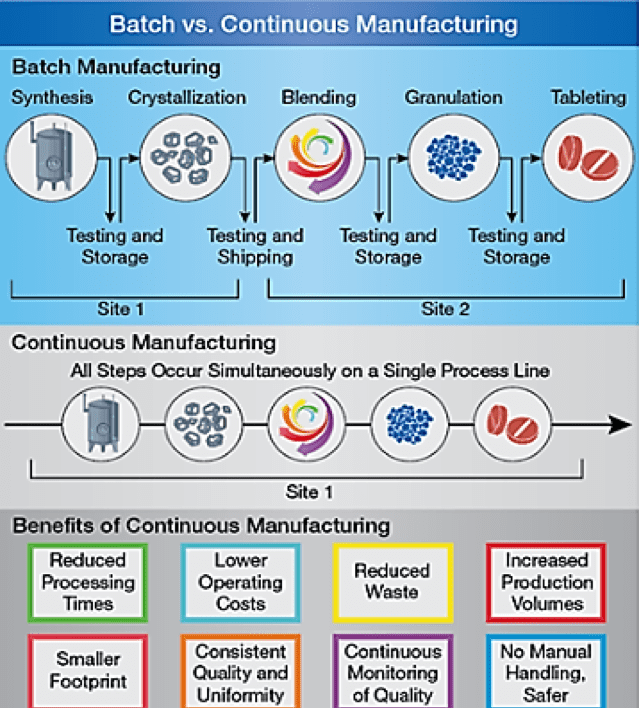
Batch manufacturing involves discrete steps. Typically, production comes to a halt while samples are tested for quality offline. During these hold times, the material may be stored or shipped to other facilities globally to complete the manufacturing process. This can greatly increase the time it takes to make drugs, adding weeks or months to the process. All this introduces risk to quality and the continuity of the supply chain. 2
In contrast, pharmaceuticals that are made using CM are moved nonstop within the same facility, eliminating hold times between steps. Material is fed through an assembly line of fully integrated components. This method saves time, reduces the likelihood for human error, and can respond more nimbly to market changes. To account for higher demand, continuous manufacturing can run for a longer period of time, which may reduce the likelihood of drug shortages 3.
Clearly, the design of a CM approach involves much of the same physical equipment used in batch manufacturing, modified to directly take products from one phase and pass it through to the next.
We must, however, put up a rather large caution sign here. The differentiation between batch and continuous processing is not this clear-cut! Let us look at some relevant examples of drugs that have simple manufacturing sequences. In these examples, multiple batching is limited and the continuous process predominates, however batching still takes place:
1) The modern manufacture of human insulin:
Several techniques are cited in the literature for manufacturing insulin. All involve recombinant DNA techniques and a variety of host cells modified to express the desired product.4
In practice, great care needs to be taken securing quantities of the desired organism for replication.
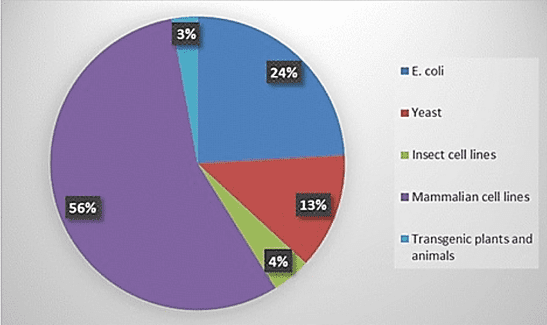
A bioreactor can be used to express the molecule by using modified bacteria such as e coli, yeast, or other living microorganisms shown on Figure 2:
Now here is how this example becomes relevant. At both the beginning of this process (cell modification to engineer insulin production). And at the end of the end (harvesting and purification), separate batches must be carefully “manufactured” and, isolated as batches! See Fig. 3 below:
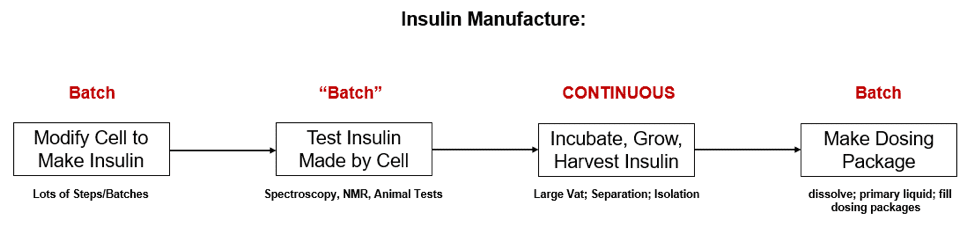
While the process is continuous in the middle, very careful genetic and purification steps must be separated. These key batching steps cannot be entirely eliminated. At least in this space-time continuum!
2) The modern manufacture of aspirin (acetylsalicylic acid):
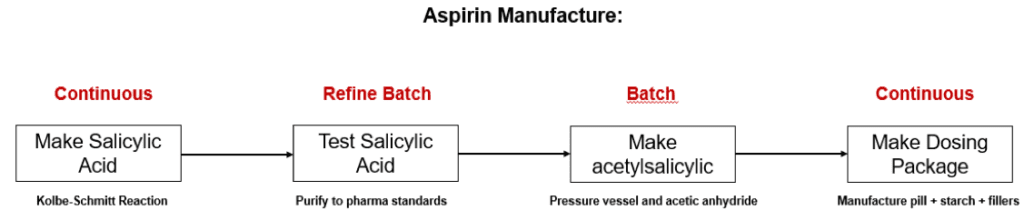
Commercially, aspirin starts out requiring a related chemical, salicylic acid, which is made under 100 atmospheres pressure combining sodium phenolate and carbon dioxide. (The Kolbe-Schmitt reaction). 5
After suitable purification to pharma standards, salicylic acid is then reacted with acetic anhydride in an acetic environment (phosphoric or sulfuric acid) to produce a powdered form of acetylsalicylic acid6. After purification, this product is mixed with corn starch, fillers, and water and pressed into tablets.
While the entire process is relatively simple, only the first and last steps shown in Fig. 4 are continuous. Batching is necessary after the salicylic and acetylsalicylic powder phases.
The author needs to emphasize two clarifying points to the above analyses.
First, these two drug examples are very common pharma products, therefore differing manufacturers may have differing methods for producing them. The author has referenced his sources, but even these sources acknowledge a variety of manufacturing methodologies and steps are used for both drugs.
Second, while the author has chosen widely-used drugs with low cost because these examples should show CM, batching is still significantly involved in both products!
Most pharma manufacturing has steps relating to isolating synthesis organisms, feedstock quality control, dosing, purity, and product quality control which directly or indirectly require batching.
While continuous manufacturing can be seen as a spreading technology, while this strategy will grow in relevance and contribute to significant reduction in manufacturing costs and higher product quality in the future, there are inherent reasons why batching will not become extinct.
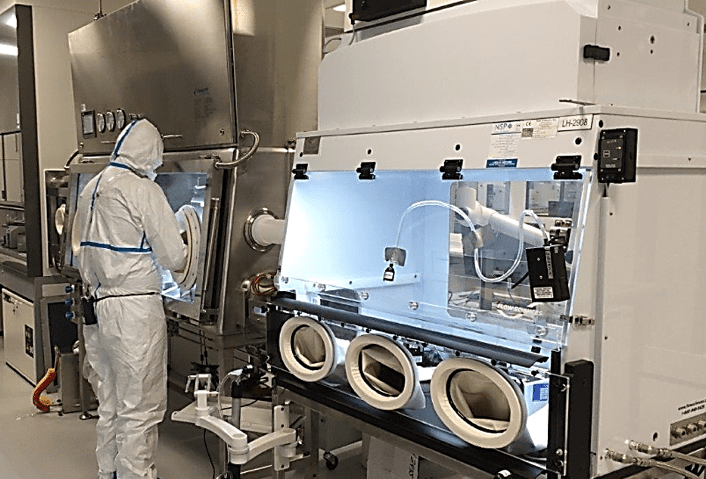
The Challenges Batching and Continuous Manufacturing Issues Bring to containment Product Design:


As figures 5&6 above show, similar units may be oriented differently to accommodate batch manufacturing (Fig. 5) or continuous manufacturing (Fig 6). Flow Sciences has been designing custom linked units similar to Fig. 6 for years as we respond to the growing demand for continuous process equipment.
Additionally, many CM applications may not ever require linking containment devices physically, but may just need hoses or other pre-made conduits added. FSI’s new Hybrid Isolator comes with several ways to field-modify installed units to include connectivity with other processes.
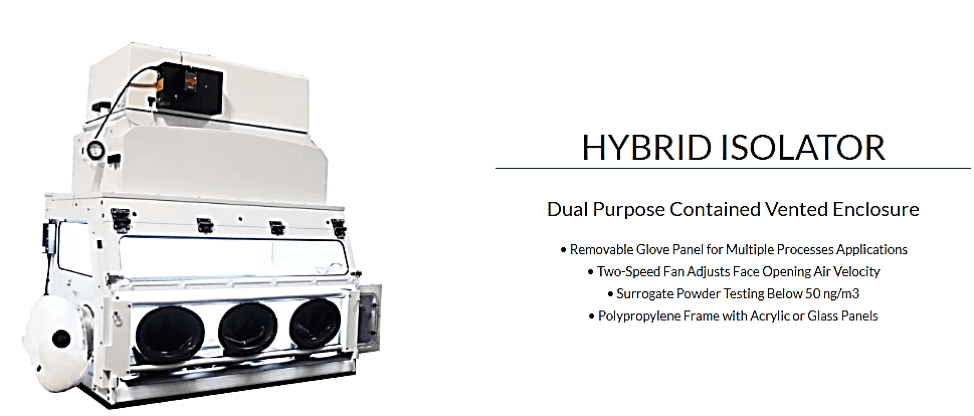
This type of product development will continue into the future as advancing CM techniques require a variety of linkages between existing containment units. The future of this industrial segment will require all containment manufacturers can furnish containment products that support transitioning manufacturing requirements.
Check out what’s already happening at www.flowsciences.com. Here’s a preview of diverse solutions for lab and manufacturing streamlining!



Commercial break: call us!
Flow Sciences 1-800-849 3429.
Footnotes:
- Wikipedia, 2019, https://en.wikipedia.org/wiki/Pharmaceutical_manufacturing
- CEP Magazine, 2018, Pharmaceutical Manufacturing: Current Trends and What’s Next
- The Case for CM, 2018, FDA Report 2018, Sou LEE, Director of Office of Testing and Research
- Microbial Cell Factories, October 2,2014, Cell Factories fir Insulin Production, US National Library of Medicine, National Institutes of Health; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4203937/
- Wikipedia, 2019, https://en.wikipedia.org/wiki/Salicylic_acid
- https://chempics.wordpress.com/2013/09/18/acetyl-salicylic-acid-aspirin/
- http://www.madehow.com/Volume-1/Aspirin.html

