Abstract:
No technology in fume hood design is more unique or stranger than the perchloric acid fume hood. This white paper describes the utility of perchloric acid in many modern applications. While perchloric acid is useful in chemistry, it is remarkably hazardous in unique ways. These hazards must be recognized, designed for, and supported with appropriate applications, equipment, and regular maintenance.
After reviewing this acid and its uses, the writer will examine questions regarding proper fume hood use and controversies which appeared recently in a Linkedin discussion of these matters by a variety of scientists involved with perchloric acid research. He will then suggest different approaches for addressing these questions.

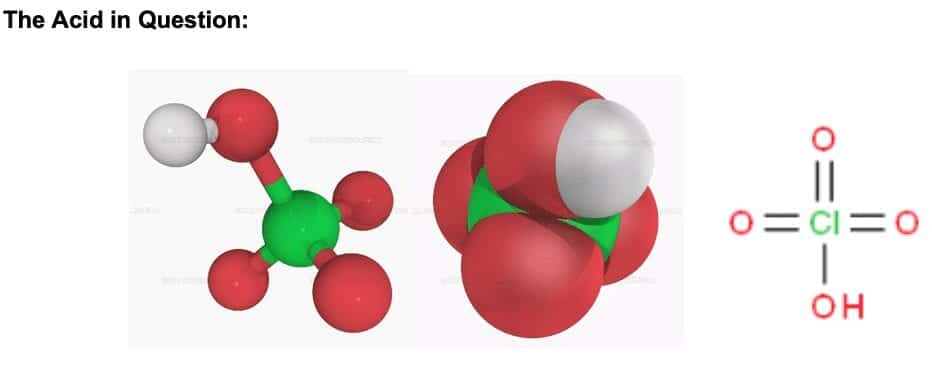
Perchloric acid, HClO4,can be symbolized in a variety of ways as shown above. It is a liquid at room temperature with a melting point of -17OC and boiling point of 203OC.
A major part of perchloric acid is the perchlorate ion, [ClO4]-1. This ion is very unstable and can, depending on reaction conditions, give off oxygen, free radicals, or combine with other reactants containing carbon to form organic peroxides.
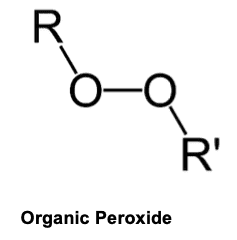
An organic peroxide is a carbon-based compound containing a “peroxy” group (two oxygen atoms joined together -O-O-). It is the double oxygen of the “peroxy” group that makes organic peroxides both useful and hazardous. The peroxy group is chemically unstable, and can decompose with varying degrees of severity.1 Many times these peroxides are explosive.
Today, ”perchloric acid is mainly produced as a precursor to ammonium perchlorate, which is used in rocket fuel. The growth in rocketry has led to increased production of perchloric acid. Several million kilograms are produced annually. Perchloric acid is one of the most proven materials for etching of liquid crystal displays and critical electronics applications as well as ore extraction and has unique properties in analytical chemistry.
In addition, it is a useful component in etching of chrome.”2
Perchloric acid in industry and in fume hoods:
Perchloric acid has a brutal history of causing industrial accidents:
“On February 20, 1947, in Los Angeles, California, 17 people were killed and 150 injured when a bath, consisting of over 1000 liters of 75% perchloric acid and 25% acetic anhydride by volume, exploded. The O’Connor Electro-Plating plant, 25 other buildings, and 40 automobiles were obliterated, and 250 nearby homes were damaged. The bath was being used to electro-polish aluminum furniture. In addition, organic compounds were added to the overheating bath when an iron rack was replaced with one coated with cellulose acetobutylrate. A few minutes later the bath exploded.” 7

While not a lab event, this tragic scene highlighted the magnitude of the danger of perchloric acid reactions with organic chemicals.
To guard against other such events, perchloric acid fume hoods and related equipment allow the safer laboratory use of this chemical in smaller quantities. Perchloric acid fume hoods use a wash-down system daily or after a procedure is complete. In most cases, this requires emptying the fume hood and running a hood-ductwork wash-down cycle of at least ten minutes. Ventilation elements of this process are shown below:

In improperly maintained perchloric acid fume hoods, white powder may appear inside the fume hood or ductwork as shown below:

Such organic perchlorate salts or organic peroxide powder residue may be explosive. Once chemical hazard specialists are summoned to analyze similar situations, they may empty the hood, run a back baffle wash-down cycle, and then gently remove any material remaining within the containment area with a sponge and water, discarding the rinse water down the wash down trench at the hood back. Other more serious procedures may be applied depending on the diagnosis of the material and other involved elements of the duct system (like ductwork problems in frame 3 above).
What the literature states about perchloric acid fume hood hazards:
- ANSI-AIHA Z9.5 2012 addresses this acid in perchloric fume hoods:

Other sections of AIHA Z 9.5 address in detail how ductwork must be made of chemically resistant materials, washed down, and not ganged together with other types of fume hoods.
The 2012 standard position on perchloric acid use shown above is much more permissive than the 2003 version of the same procedure outline which is stated below (slightly different number for same section):

Note that the newer standard does not regard perchloric acid as a peroxide fume-former at lower temperature if the concentration is less than 72% because its vapor pressure in lower concentrations is too low to cause fume release at room temperature. The new standard primarily focuses on the acid’s inherent vapor release properties and reactions known to be hazardous in lower concentrations.
We need to understand the implications of this important change. The author reviewed other sources of information regarding lower temperatures and peroxide presence.
It turns out, other sources in the literature significantly differ with the “new” section 3.2.5 in their view of perchloric acid procedures and which ones pose a threat to safety.
- The University of Calgary states a more cautious approach to perchloric acid at concentrations less than 72%. In its formal perchloric acid procedure5, the following is noted:
”Perchloric acid (60-72%) acts and reacts with alcohols and certain other organic compounds to form very unstable perchlorate esters at room temperature.”
Other reactions with lower concentrations of perchloric acid are also mentioned in this document.
- The University of Illinois has published a background paper on its website citing three known conditions where concentrations of perchloric acid less than 72% can cause problems 6.
- Perchloric acid forms an azeotrope with water at a concentration of 72.5% perchloric acid. Therefore, aqueous solutions do not form anhydrous perchloric acid by evaporation. However, dangerous anhydrous perchloric acid can form when an aqueous solution is subjected to strong dehydrating conditions such as exposure to concentrated sulfuric acid, acetic anhydride, or phosphorous pentoxide.
- At elevated temperatures, vapors from perchloric acid can condense on surfaces in the ductwork of the hood, where they form perchlorate salts that are often highly shock-sensitive and that pose a serious explosion hazard.
- Perchloric acid reacts with alcohols and certain other organic compounds to form highly unstable and explosive perchlorate esters.
- In A Guideline for the Use of Perchloric Acid and Perchloric Acid Fume Hoods, The British Columbia Ministry of Energy and Mines concludes: “A fume hood designated for the use of perchloric acid must be used when handling or performing a reaction with perchloric acid. There are risks of fire and/or explosion should perchloric acid contact incompatible materials or perchloric acid vapors, released by the heating of perchloric acid, condense and crystallize on laboratory surfaces. Using a proper perchloric acid fume hood with a wash-down system is imperative in preventing inadvertent contact with incompatibles and the formation of perchlorate salts. Perchlorate salts are highly explosive and sensitive to shocks and vibrations, including the normal working vibrations of a fume hood.”
This conclusion is definitive, affirmative, and emphatic. No qualifiers based on the concentration of perchloric acid.
The author’s conclusion: As a manufacturer of perchloric acid fume hoods, Flow Sciences recommends all perchloric acid be used inside a perchloric acid fume hood unless the user of such a hood (and institution) can definitively assure conditions during the procedure afford no risk. Risks are present when perchloric acid is heated or when several types of reactions produce organic peroxides. For these reasons, the author takes strong exception to the 2012 revisions to Z 9.5. We particularly disagree with the omission of this 2003 phrase from the later 2012 document:
“The immediate supervisor and institutional/corporate responsible person (e.g. Safety officer/Chemical Hygiene Officer) always should be notified before these substances are used.”
While the vapor pressure of the perchloric acid at room temperature is not significant in solutions of less than 72%, aerosol electrochemical or digestive reaction products may dry to perchlorate salts or other materials which may collect as a powder inside the fume hood and ductwork. Such deposits may be explosive!

Conversations online regarding whether a perchloric acid hood is really necessary:
These conversations occurred on my Linkedin page during the week of February 11, 2019:
- Two researchers commented after I aired a video on a functioning perchloric acid fume hood in late January9:
- I personally think DUCTLESS FUME HOOD is better. It’s cost efficient, the carbon can also filter poisonous fumes. The filters are actually changed to curb that, and at the same time I am also making my opinion from cost efficiency. I am willing to learn more from an experienced professional though.
- Perchloric acid fume hood… Interesting!!! I’m currently using Alkaline fume hood that neutralizes acidic fumes.
Author’s comment: Unless very dilute concentrations of perchloric acid are being used, the author does not recommend the use of ductless activated charcoal filters for perchloric acid procedures. Being a pure carbon source, the charcoal may capture perchloric acid fumes or aerosols and form organic peroxides with the charcoal filter material itself! Such a reaction could be a redox reaction independent of the acidity of the ingested material. We recommend a careful analysis of this possibility before using the technology outlined by both researchers.
- A researcher needed more clarificationon when perchloric acid hoods are needed and how they should be exhausted.
Hi Robert, Good Day, Perchloric Acid Fume Hood Performance Demo was really good. We have following doubts and need clarification. Is it necessary to use separate fume hood for perchloric acid applications? If we are using very minimum qty. of perchloric acid does the fume hood really need wash down systems?
Author’s comment: Whenever perchloric acid use involves very dilute concentrations, the author recommends careful review by Health & Safety before such acid is used in a standard fume hood. Be mindful that lab research or procedures may changeand invalidate any rationale used to avoid perchloric acid fume hood technology.
To the additional concern regarding exhaust exclusivity, most published authorities on this matter believe that the perchloric acid exhaust system should always be kept separate from other chemical exhaust streams.
The British Columbia Ministry of Energy and Mines8 states:
”Ductwork for perchloric acid hoods and exhaust systems should take the shortest path to the outside of the building and should not be in the same manifold as other exhaust systems. Horizontal ductwork should be avoided as it creates difficulties for drainage and spray coverage. If unavoidable, horizontal runs should be as short as possible, with no sharp turns or bends, and sloped to ensure drainage.”
The possibility that perchloric acid fume hood exhaust in a mixed exhaust stream could react with fumes and residues from non-perchloric reactions is worth avoiding at all costs. 8

- A prominent international pharmaceutical company’s Procurement and Environmental Health and Safety officer emailed me the following question: “As you are probably aware ANSI Z9.5 – Laboratory Ventilation, 3.2.5, requires perchloric acid fume hood when handling anhydrous perchloric acid >85%. Also the requirements under NFPA 45 – 2015: Standard on Fire Protection for Laboratories Using Chemicals, 12.1* Perchloric acid heated above ambient temperatures shall only be used in a chemical fume hood specifically designed for its use…“
Does Flow Sciences recommend this fume hood for applications using concentrated perchloric acid (>72%) and/or heating perchloric acid only? Would you recommend a normal chemical fume hood for use in situations such as preparing dilutions of 4.0ml of 70% Perchloric Acid in 4L of water about once a month?”
This question is entirely justified and arises out of the new language set forth by AIHA Z9.5 already discussed. The officer even copied the newer version of section 3.2.5 into the email inquiry.
Author’s comment: This legitimate question actually turned this white paper into a different and more focused direction! I am grateful the issue was put to me in such an inescapably direct manner.
My answer to this question is therefore also the summary of the entire white paper.
- I believe ANSI AIHA Z9.5 2012 section 3.2.5 is a step too far in its implied recommendations regarding perchloric acid fume hoods. It is true that the standard requires 85% or greater concentrations of HClO4 shall always be used in a perchloric acid fume hood. (3.2.5 left column). It is also true that the standard recommends perchloric acid digestions (no concentration mentioned, right column) be conducted in perchloric acid hoods. It is also true that an opened bottle of 70% perchloric acid at 20o C cannot evolve gaseous HClO4 in quantities capable of reacting with incompatible organic compounds.
No claims are made in the right or left column regarding any other issues involving dilute or concentrated HClO4.
- So what’s the big deal? The standard is carefully written so that AIHA offers no opinion or recommendation on any other perchloric acid application outside of heating, digestion procedures, or opening a bottle of 70% HClO4 and observing it. Consequently, where is the larger responsibility to designate containment equipment for all remaining reactions? ANSI/AIHA/ASSE Z9.5-2012 currently sets forth no guidance whatsoever on this.
The AIHA Z 9.5 2003 Standard did offer guidance. It was clearly stated in the right column:
“The immediate supervisor and institutional/corporate responsible person (e.g. Safety officer/Chemical Hygiene Officer) always should be notified before these substances are used.”
Even though this “should be” recommendation has not been placed in the 2012 version of 3.2.5, the author believes liability for accidents will inevitably become the legal responsibility of those in charge at involved facilities. As a manufacturer, Flow Sciences continues to believe standard hoods should not become the home for the majority of perchloric acid procedures not now addressed by ANSI AIHA Z9.5 2012. Applications involving perchloric acid should be rigorously examined and past experience heeded before any perchloric procedure is undertaken in a standard chemical fume hood. SOP’s should be agreed to and circulated. Signage should be agreed to and posted. Maintenance should be regular and documented. And most importantly, those supervisors assigned responsibility for such experiments and procedures should monitor and supervise all operations closely.
Anything less puts the administrator and staff at increased physical and legal peril!
One more photographic comment is necessary to summarize this paper. We began by reviewing the uses of perchloric acid. Among these uses, the most prominent is production of ammonium perchlorate, an ingredient in solid rocket fuel. Here are three photos of the PEPCON chemical plant in Henderson Nevada before and after a small fire ignited part of the Ammonium Perchlorate being stored there.

Safety specialists must get the chemistry right in perchloric acid research and production. The perchloric acid fume hood and its use are of primary importance to this mission. Those who manufacture lab safety products, promulgate safety standards, and enforce them must work together toward this goal.
Footnotes: (Italics with accompanying footnote indicate a direct quotation from the cited text)
- https://en.wikipedia.org/wiki/Organic_peroxide
- https://en.wikipedia.org/wiki/Perchloric_acid
- http://www.fabricatedplastics.com/case/perchloric-acid-scrubber-system/
- Excerpted from twin City Fan and Blower Brochure, 2012, Minneapolis, MN.
- Perchloric Acid Fume Hoods, Revision, University of Calgary, 2/7/2003
- https://www.drs.illinois.edu/SafetyLibrary/PerchloricAcid
- https://en.wikipedia.org/wiki/Perchloric_acid
- A Guideline for the Use of Perchloric Acid and Perchloric Acid Fume Hoods, British Columbia Ministry of Energy and Mines, 2016
- https://www.linkedin.com/in/robert-haugen-22918546
- https://sma.nasa.gov/docs/default-source/safety-messages/safetymessage-2012-11-05-pepconexplosion.pdf?sfvrsn=ceae1ef8_6

