Dr. Bob Haugen
Director of Product and Technology Development
Gary Dean
Mechanical Designer
What happens when the air itself becomes a threat to whatever process is being undertaken?
Abstract:
There are frequent cases where the atmosphere we live in every day can become a laboratory hazard. The earth’s atmosphere contains Nitrogen (78%), Oxygen (21%), Argon (1%), and Carbon Dioxide (.04%)1,2.
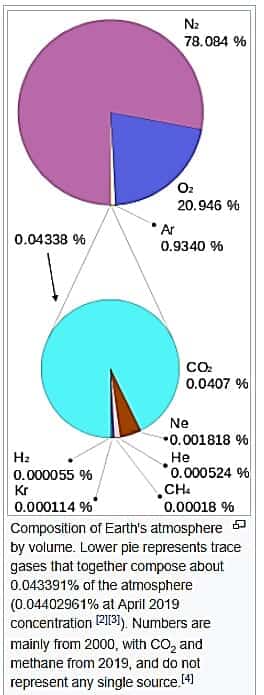
Any chemist knows a 21% Oxygen atmosphere supports combustion. Our planet has many materials on its surface that are oxidized without remorse by Earth’s atmosphere: iron, copper, and to a much more dramatic extent, carbon-containing compounds including wood, coal, organic solvents, and human flesh.
In thoughtfully designed laboratories, the containment device atmosphere should never cause unintended and un-measured chemical reactions with the procedure under way.
Typically, lab containment devices use air flow to control fumes by “pushing back” vapors away from the device’s access opening. What happens when the air itself becomes a threat to whatever process is being undertaken?
In this paper, the author will detail how a Flow Sciences Nitrogenema (END) device works. Applications will be discussed. Advantages in safety, utility, and product purity will be reviewed. There are many potential uses for such devices; some examples will be discussed. 3
Discussion:
The Nitrogenema© (END product series) is a fanless unit designed for low moisture or low oxygen procedures. It utilizes a nitrogen feed system to replace oxygen and water vapor with an inert, less reactive atmosphere.
This family of enclosures is designed to offer protection of product and personnel from environmental components such as water vapor (humidity) and oxygen, both significant constituents of the air flow typically applied to contain chemical reactions. The standard END enclosure is constructed from static dissipative clear acrylic upper sections and a phenolic resin base. Since such an enclosure eliminates humidity by design, the static dissipation characteristics of the cabinet greatly reduce the likelihood of internal sparks inside this very dry environment.
These enclosures have a connection point for introducing minimally reactive gases, such as nitrogen or argon. Other attributes of this system include a pressure relief valve, a pressure gauge, integrated glove ports, and a pass-through for transferring products and equipment into and out of the enclosure. Optional components include a power outlet located inside the enclosure and a gas flow controller which uses feedback mechanisms to minimize unwanted gases like oxygen and water vapor by continually replacing these gases with dry nitrogen or other gases3. Figure 1 shows the key components of this system:
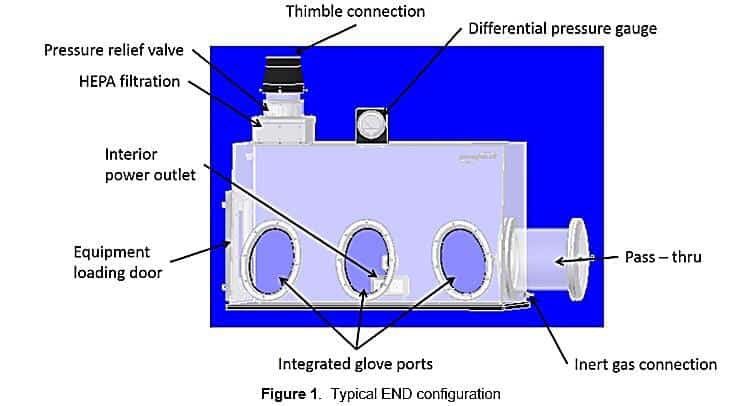
The nitrogen supply can come from a variety of sources. Flow Sciences can provide a nitrogen flow controller which has programmable logic that regulates the flow of inert gas into the enclosure using either relative humidity (RH) or oxygen concentration as defining parameters for control set points. This controller is shown in figure 2.

A relief valve is designed to bleed out the internal END atmosphere as additional dry gas is added by the controller when required. There is a slight overpressure inside the cavity of about 0.3 to 0.7 inches WC during this cycle. The gas exiting the END is HEPA-filtered before passing into a thimble connection to house exhaust. (While nitrogen is not toxic, exhaust containing large amounts of nitrogen discharged directly into the laboratory air could displace oxygen in the room air and cause issues)
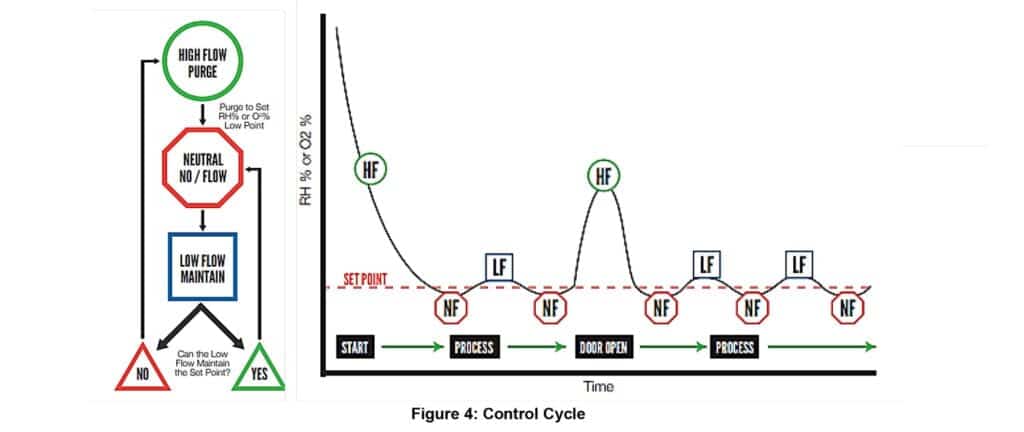
The controller detailed above must perform three functions:
1) When the sensed environment has a widely different oxygen or humidity value from that desired, the inert gas is allowed to flow at maximum rate. This will not cause over-pressurization of the END because the relief valve and filter allows the exhausted gas to leave the END device.
2) When fine adjustment of the environment is needed, a low flow setting allows the inert gas to flow at a lower rate.
3) When the desired environment has been obtained, the flow of the inert gas into the enclosure is stopped until increased oxygen or humidity is again sensed, requiring automatic re-adjustment by more inert gas flow.
This passive replenished-flow system yields a very stable end point over a short time; see figure 3 below:
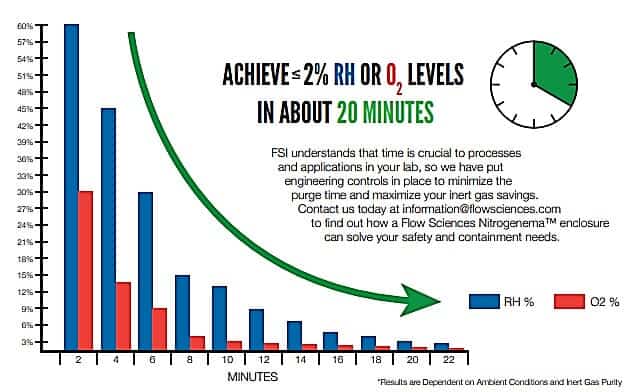
Additionally, once a control level is reached, an appropriate unreactive gas feed control system will produce a stable low oxygen/water vapor concentration inside the containment cavity. (figure 4)
Flow Sciences has supplied this product to customers with the following needs:
1) Manipulation of pyrophoric compounds which might oxidize or ignite in the presence of atmospheric oxygen.
2) Weighing or manipulation of Hygroscopic substances which can chemically combine with atmospheric water and not even allow precise weighing because of this characteristic. These products include pharmaceutical products which are dispensed into bottles with desiccant packages inside.

3) Dispensing pharmaceutical products into smaller packages with atmospheric integrity

4) Nanotube production and packaging

5) Testing food product packaging in its ability to protect foods susceptible to atmospheric oxygen attack (Wine, cheese, some fruit products)
Flow Sciences has even used these units with modified systems and materials of construction to accommodate special applications as shown below.
- Different systems for water and oxygen level control.

- Different Materials of construction and Antechambers for special applications.
- a) Multi-stage unit with antechamber

- b) Coved stainless steel and glass for easy cleaning

Conclusion:
The END Nitrogenema is an artificial atmosphere low-flow glove box with a variety of applications. All applications require the removal or suppression of oxygen, water vapor, or both. This is achieved by introducing a minimally reactive gas in quantities large enough to flush out atmospheric water vapor and oxygen.
This approach has been used in a variety of applications, materials of construction, and accessories. Flow sciences has found a rather substantial market for this unit and anticipate its wide application in years to come. Please contact Flow Sciences for more information if you foresee an application in your facility!
This unit comes in many sizes and materials of construction. The END provides ultimate product protection at an affordable cost. We will be happy to discuss your application. Any custom unit you specify will be tested per your application and we will ship it only when it meets customer specification. We do this all the time!
References:
- https://en.wikipedia.org/wiki/Atmosphere_of_Earth
- Plus several other gases in small quantities: https://en.wikipedia.org/wiki/Atmosphere_of_Earth
- Argon and possibly other non-reactive gases have also been used

