

Dr. Bob Haugen
Flow Sciences Inc.
10/15/2020
Abstract: Biosafety cabinets have been around for generations1. These cabinets provide an enclosed, filtered, and ventilated lab workspace for materials containing pathogens requiring a defined biosafety level. Several different types of BSC’s exist, differentiated by their biocontainment levels and construction.
Once any type of containment device presents itself in the marketplace, regulatory agencies and associations begin looking at this new product with the intent of establishing standards. As manufacturers, we accept industry standards, rapidly organizing our own products within the established scope of the standard.
However, because equipment used in biological research changes constantly, established containment guidance and standards can become out of touch with the evolving art. To this point, the writer believes biosafety containment standards need reexamination. The NSF 49 biosafety cabinet standard cannot address all evolving experimental and production realities by itself.
Things have changed.
They are about to change some more.
The History of NIH, CDC, NSF, and Standard 49 6:
- Biosafety Cabinets before NSF 49
The National Institutes of Health (NIH) and the Center for Disease Control (CDC) began working with ventilated cabinets in the mid 1950’s. As projects increased in frequency and complexity, the types of containment equipment became more defined.
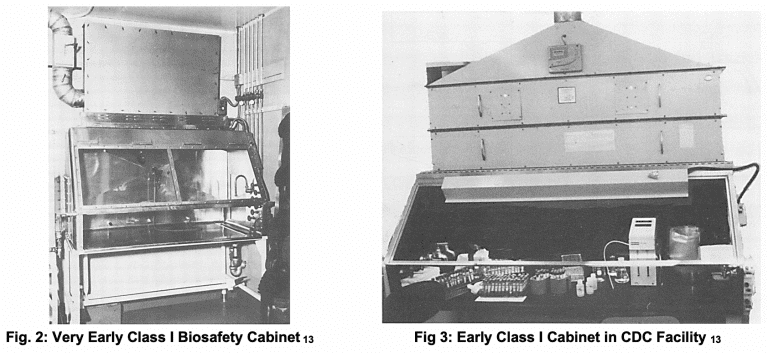
Class I cabinets generally exhausted contents to the outside of labs through newly developed HEPA filters. They offered no product protection since incoming laboratory air was directly used to ventilate the containment chamber.
Class II cabinets were designed to introduce filtered air into the cabinet and to exhaust filtered air to the exterior of the building or re-introduce it back into the lab (product and personnel protection).
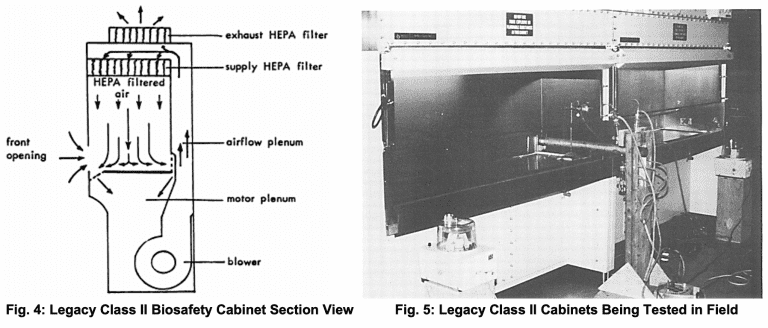
While these early examples of biosafety cabinets showed great promise, NIH was highly concerned about labs under its control having poorly operating units with bad air flow 13. These early devices were not terribly consistent in performance and had other problems, including servicing and recertification.
Kruse and Coombs report 13, “In 1985, 20 examples of improper certifications were observed over 13 years of experience. [Improper certification or a lack of knowledge] of various cabinets can result in serious problems.
As a result of this study and other unsatisfactory containment problems at NIH facilities, a search for some harmonized material and performance standard administered by an independent organization was initiated.
In 1974, NIH issued NIH 112-03c, a specification for a Class II biosafety cabinet 17. This materials and performance specification would ideally be a blueprint for a standard Class II product/personnel protection containment device.
While some interest in the above NIH specification was evident, the initial interest in manufacturing this specified product was limited and the price was high among bid participants. NuAire built a prototype, while many other companies insisted the government buy their proprietary biosafety products instead.
NuAire was a fledgling company when NIH visited the garage where the new cabinet had been built in 1974. NIH research in this era was primarily focused on tissue culture 14,15,16 et al. Product and personnel protection had been emphasized in contemporary specifications primarily to assure containment integrity in the tissue culture area of research.
The NuAire visit interested NIH because their prototype seemed to conform with NIH 112-03c requirements for symmetric, laminar air downflow patterns through the cabinet. Both personal and product protection were perceived as possible. Minimal cross-sample contamination within the containment cavity was also probable.
While the science appeared to be congealing, NIH realized it would need to standardize a cabinet design through an independent agency to ensure product standardization and attainable performance throughout the industries manufacturing biosafety cabinets. Once such independent standards were attained by several manufacturers, the government could easily attract bids on comparable products in a competitive bid marketplace.
- NSF 49 Development of Class II Biosafety Cabinet Standard
NSF became the third-party independent agency which NIH contacted to do this work.
NSF International (Previously known as the National Sanitation Foundation) was founded at the University of Michigan School of Public Health in 1944 2. Initially, the Foundation focused on sanitization standards for foodstuffs requiring health and safety regulation. The process used to develop the very first NSF standard covering soda fountain and luncheonette equipment became the process by which NSF International developed subsequent public health and safety standards.
Items covered by NSF standards now include:
- Pools and Spas
- Plastic piping
- Wastewater treatment
- Water treatment and distribution
- Bottled water
- Drinking water additives
- Dietary supplements
- Pharmaceutical training and auditing
- Seafood Quality
- Kitchen products and appliances
- Pharma and Biotech: Biosafety cabinets under NSF 49 4.
The biosafety cabinet classification system (#11 on the above list) is memorialized in Centers for Disease Control and Prevention, National Institutes of Health, Biosafety in Microbiological and Biomedical Laboratories; 5th Edition 10. Today, this one standard for common Class II biosafety cabinets predominates.
Quoting from Appendix A, Section VII of the above-mentioned reference:
“National Sanitation Foundation (NSF) Standard #49 for Class II BSCs was first published in 1976, providing the first independent standard for design, manufacture and testing of BSCs. This standard “replaced” the NIH specifications, which were being used by other institutions and organizations purchasing BSCs. NSF/ANSI Standard 49—2007 incorporates current specifications regarding design, materials, construction, and testing. This Standard for BSCs establishes performance criteria and provides the minimum testing requirements that are accepted in the United States. Cabinets, which meet the Standard and are certified by NSF and bear an NSF Mark.
“NSF/ANSI Standard 49—2007 pertains to all models of Class II cabinets (Type A1, A2, B1, B2) and provides a series of specifications regarding:
- Design/construction
- Performance
- Installation recommendations
- Recommended microbiological decontamination procedure
- References and specifications pertinent to Class II Biosafety Cabinetry”
The NSF was contacted by NIH to provide liaison services among industry, regulatory agencies of government, professional and technical organizations, and consumer representatives in developing a standard that would be agreeable to users and manufacturers of class II biosafety cabinets. 13
The 1976 NSF 49 Standard for Class II, Type 2 Biosafety cabinets minimized design diversity between various containment products by mandating construction and performance criteria. U.S. government requirements for design uniformity and standardized research within its own tissue culture research infrastructure was the predominant reason NSF 49 was accepted by NIH as its institutional biosafety cabinet standard.
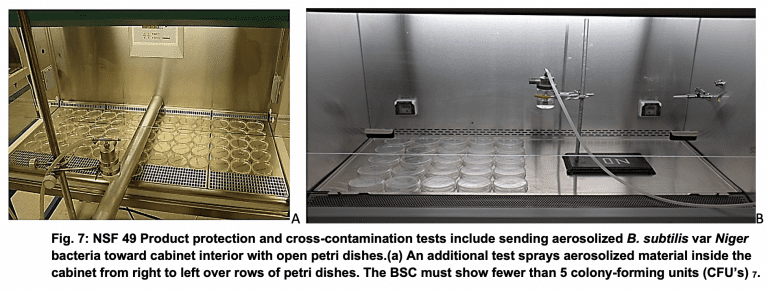
It is important to note that although the first NSF 49 standard was published in 1976, the first NSF list of compliant manufacturers was not issued until February 1978 13. This time delay was necessary to allow various manufacturers to work with NSF to iron out design discrepancies and NSF 49 wording to harmonize products available with the new standard. A final round of changes generally made for these purposes, was made and a revised NSF 49 Standard published in November 1978.
NSF continues updating this standard approximately every 10 years. In addition, NSF updates the list of Class II cabinets that meet the requirements of NSF 49. This list, available online from NSF, is generally used to verify manufactures are eligible to quote, construct, test, and install biosafety cabinets. 13
In addition to establishing construction and material standards, NSF 49 also created quality control and field certification tests. A “listing program,” was also set up (eventually online) where CFM and velocity set-up parameters for each BSC brand were listed to assist the efforts of field certifiers. 3.
In 2016, a subsequent version of the standard was designated NSF/ANSI 49-2016, reflecting its listing by the American National Standards Institute (ANSI)5.
NSF 49 has many detailed materials and performance tests. Any Class II biosafety cabinet attempting certification is required to face and pass all such tests. Some of these specified requirements are listed below:
- Interior work surface shall be 300 grade stainless steel
- Double wall construction with a mid-pressure interlayer
- Corrosion-resistant interior surfaces required
- Laminated or Tempered glass glazing materials, plus alternate materials specified
- Bending radii for interior steel liner parts specified
- No exposed screws permitted on interior surfaces
- Gasketed exterior surface screws
- HEPA or ULPA filters of appropriate type and efficiency
- Leak tests and any required pre-shipping patching techniques specified
- Proven Cabinet performance, including:
- Pressure decay 30 minutes <10% at pressure of 2” wg. above atmospheric.
- Verify HEPA leak with DOP to <0.01% of upstream amount
- Sound level < 67 dBa under test conditions
- Average lighting 60 foot-candles or greater
- Fan vibration maximum specified
- Personal, product, and cross-contamination per specified tests:
- Certificated third party UL 61010-1 passing performance required. (Entirely separate test by another testing agency)

Emerging Challenges to NSF 49:
- Evolving Biosafety Cabinet Needs
As time has passed, NSF 49 standardized tests and material requirements have become less easy to apply to contemporary containment needs in biological research. Production needs for containment devices have also clashed with NSF 49’s tissue culture model.
Covid 19 and its gigantic impact on the evolution of modern containment are typical examples of these changes. Treatments and vaccines for the Pandemic have grabbed the highest priority in research and pharma production. Research on more traditional pharma projects has cooled down as staff and equipment move into the Covid 19 arena. To research diseases and produce vaccines and other medicines in large quantities, more complex containment equipment with irregularly shaped interior spaces and access openings has become the rule rather than the exception. These shapes and production demands have also spurred a reexamination of lateral, rather than the downward flow units upon which NSF 49 is based.
The Scientist7 recently discussed pharma’s response to Covid 19:
Many of the (Pfizer) staff working on COVID-19 projects have been reshuffled from some of the hundreds of clinical trials that drug companies have been forced to put on hold due to the pandemic. In March, Lilly announced that the start of most new studies would be delayed and that new enrollment in ongoing studies would be halted. According to Skovronsky, many of the staff who would have been working on these trials were re-deployed to work on the COVID-19 research instead.
Other companies are shuffling staff around in a similar way. Owlstone’s Boyle says his company’s stalled trials freed some employees and resources to focus on COVID-19. ‘We’re trying to make sure that we can still deliver on those core programs,’ he adds. ‘But [we’re] redeploying the resources into the specific areas of the COVID problem in the near term.
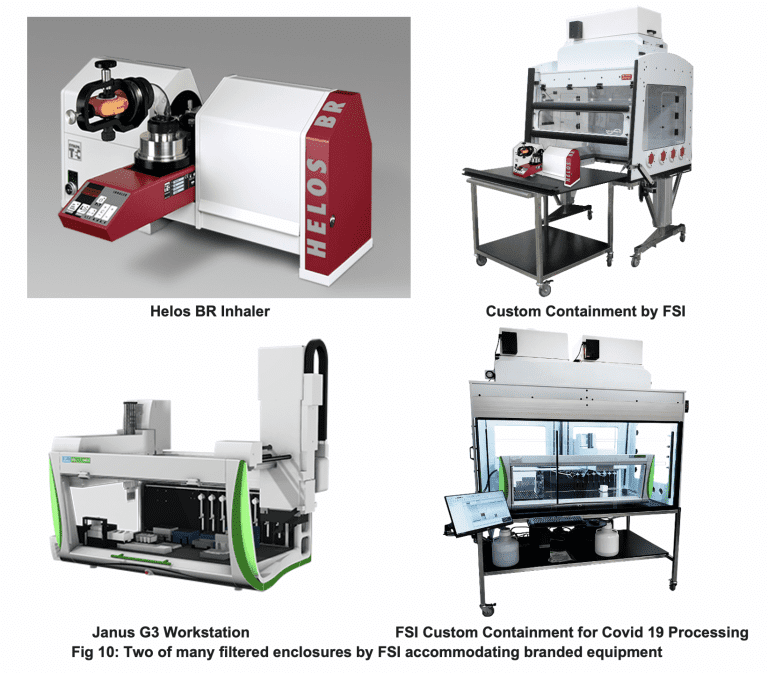
Today, redeployment and re-tasking are affecting pharma personnel and equipment purchases as well. Flow Sciences is receiving many requests for specialized containment to house branded, specific automated equipment placed inside containment spaces. This significant demand is arising from needs in vaccine research and pharma production in general.
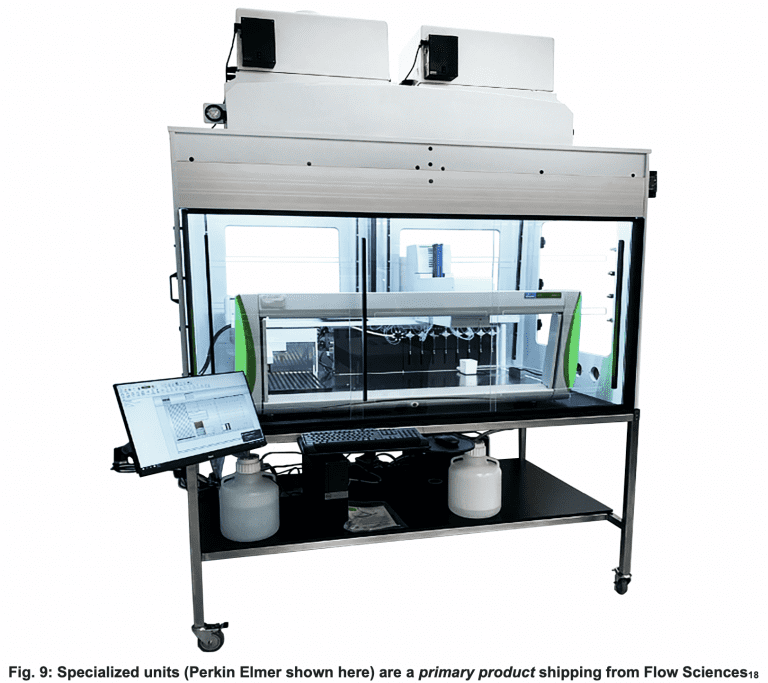
Figure 10 below depicts two examples of specified specialized equipment inside customized containment cavities. The equipment is typically complicated in shape, requiring a specified clean operating environment. Simultaneously, worker safety and environmental purity in the employee workspace must be provided.
In pharmaceutical environments, these requirements operationally define containment.
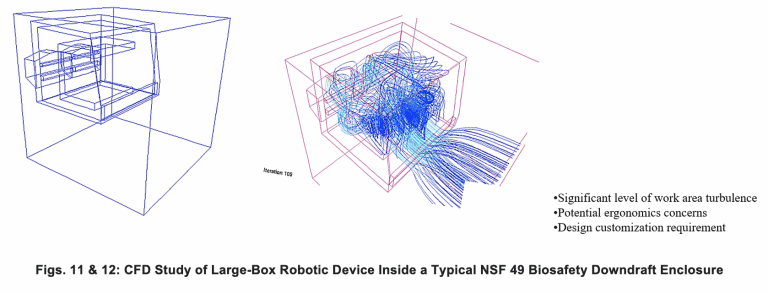
Many times, functioning solutions cannot be achieved without a complete revamping of the airflow through the containment area. Figures 11 and 12 show how standard downflow Class II Type 2 cabinets can produce chaotic turbulence when large objects are placed within their confines, substantially blocking smooth downflow. Figure 13 shows what lateral flow, as opposed to downflow air, can do to smooth out internal flow with certain problematic shapes, improving containment (CFD Flow Diagrams by FSI in all cases).
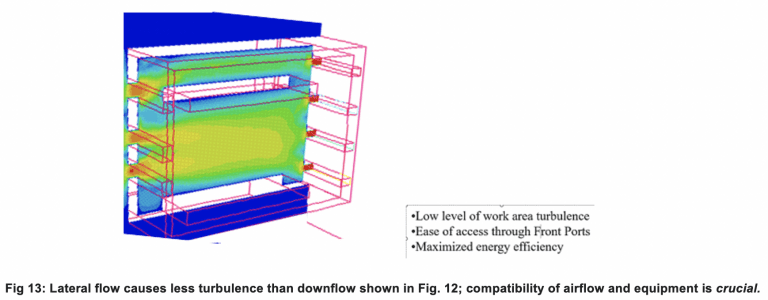
Other bulky equipment like balances, grinding mills, and shakers also work well in lateral flow applications.
- New Imperatives for Containment Testing
For all the historical and contemporary reasons discussed above, we have a need to test contemporary devices in ways different from NSF 49. The reason to test non-standard containment devices is to verify product and personnel protection per facility standards. When urgent performance validation and production timelines become compressed, demonstrating safety and purity within research and production deadlines must remain the top priority. “Warp speed” requires we use time wisely, testing containment required for personal safety under specific conditions and circumstances.
This trend will require customized testing. While NSF 49 will not disappear, certification and testing will significantly and permanently move toward specific tests that mirror the applications carried out inside the containment area.
We will know if it works when it works.
Not when we place these units into traditional, standardized obstacle courses!
The challenges to NSF 49 in this changed environment are significant. NSF 49 mandates a rigorous rectangular cuboid geometry which served tissue culture procedures well. Today, such geometry is not helpful in many rapidly evolving pharma applications. Larger irregular containment cavities that match applications are becoming the rule rather than the exception. Spreading petri dishes over an entire surface interior as contamination detectors only vaguely represents contemporary applications used in biosafety containment.
Figures 14 and 15 below highlight these realities. Figure 14 shows some key equipment used to test Type II biosafety cabinets per NSF 49. Traditional stuff.
Figure 15 shows some of the equipment commonly used in pharma research and manufacturing today. These items are not included and may not have been invented when the NSF 49 test was introduced. Many depicted pieces in figure 15 represent significant kinetic internal aerodynamic challenges, which may uniquely affect containment.
Newer stuff.


In summary, NSF 49 Standards are limited, in many cases providing equipment users with two safety options: 1) Chose an establish and certified NSF 49 product and take chances with irregular equipment and movements outside the scope of NSF 49 testing, or 2) Chose a completely unique custom product which, by definition, will not follow NSF 49 guidelines, but may be application-tested using other means.
Today, the writer sees the second option as more practical and predictive.
What kind of containment test is appropriate in this evolving reality?
- Achieving demonstrable containment with irregular, purpose-built equipment
The writer believes surrogate testing is one very useful technique for evaluating complex biosafety equipment. Such an approach is already established, formalized, and accepted in the literature.
Surrogate testing sets up actual process equipment inside the containment device to be tested. Grinders grind, shakers shake, rotovaps rotate using surrogate, traceable chemicals resembling the toxic materials destined for field use. The most frequently used test for this type of validation is outlined in ISPE Good Practice Guide: Assessing The Particle Containment Performance of Pharmaceutical Equipment 8.

All sampling in this Good Practice Guide is performed in accordance with methodologies published in Section II, Sampling, Measurement, Methods, and Instruments, of the Federal Occupational Safety and Health Administration (OSHA) Technical Manual; and the ISPE APCPPE Guideline.
This Good Practice Guide suggests methods for benchmarking procedures and improving them. The methods support acquisition of data associated with handling of pharmaceutical ingredients that can be useful in the assessment of potential risks. To directly quote from the Guide’s Introduction 8:
“The revision was undertaken to allow the Guide to address a broader selection of containment technologies and processing equipment than those covered in the first edition of the Guide.
The containment capability of equipment is an important factor in evaluating the risks associated with the handling of pharmaceutical ingredients; of specific interest are:
- The potential exposure of the operator
- The potential for uncontrolled release of pharmaceutical ingredients within the facility
- The potential exposure of the outdoor environment “
In general terms, this Guide addresses containment of materials under operational use.
We here include three illustrations of unique containment devices. They have been tested by Flow Sciences for containment (personal protection), for internal air purity (product protection), and two of the three for both. The manufacturer takes its lead from our customer on capabilities and performance under their anticipated conditions.
- Surrogate Testing Examples:
The following testing examples demonstrate how surrogate testing is carried out and why more is usually learned from this testing philosophy than from standardized testing.
- Model CQ11313
Containment Challenge:
In this study, naproxen sodium, an industry accepted surrogate powder was utilized to determine the containment expected for compounds during normal work practices. (Powder retrieval, 3.45-gram weigh, water dissolution, appropriate cleaning and tareing)
All sampling was performed in accordance with Methods and Instruments, Federal Occupational Safety and Health Administration (OSHA) Technical Manual.
Surrogate Materials: Naproxen Sodium, SF6 (g) (ASHRAE 110 and HAM tests)
Specified Containment Requirement: < 5 ng/m3 or less naproxen sodium in breathing zone and in lab area; SF6 < 0.05 ppm in ASHRAE 110 and HAM challenges.
Results: all readings < 0.9 ng/m3 for sodium naproxen and < 0.01 ppm for SF6.
All test results are well below the specified containment requirements.

2) Model CQ 4790: a complex process array for a prominent US pharma company:
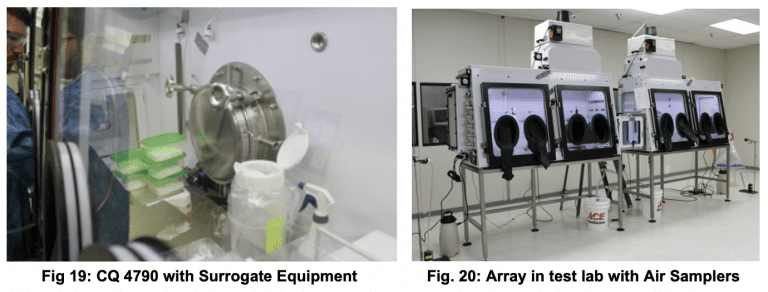
Containment Challenge: All sampling was performed under the direction of an IES Certified Industrial Hygienist (CIH) in accordance with Best Industrial Hygiene Practices and Methods and Instruments, of the Federal Occupational Safety and Health Administration (OSHA) Technical Manual APCPPE
Surrogate Materials: Naproxen Sodium
Specified Containment Requirement: 50 ng/m3 with internal Class 5 Interior Cleanliness
Results: All readings < 0.356 ng/m3 with Class 5 Cleanliness level (product protection).
3) Testing for product and personal protection using a Glovebox Workstation.

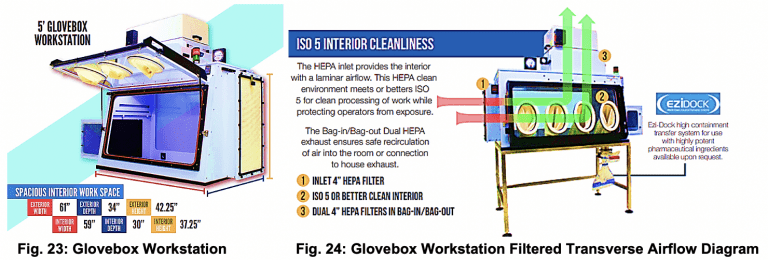
Containment Challenge: Evaluate the effectiveness of the CQ04160 during vial transfer operations and compare to a biosafety cabinet using the same process.
Class 5 interior air purity to be maintained.
Surrogate Materials: Approximately 1,084g of Pharmatose® 450M
Specified Containment Requirement: .050 mg/m3 (nanograms per cubic meter of air) time weighted average
Results: 0.011 mg/m³ in worker breathing zone & proximate work area
As the reader can determine from a review of these three examples, surrogate tests follow a format, use established procedures, and must demonstrate specified equipment performance under the known conditions of use.
Is this equipment useful in other applications?
Maybe.
Nevertheless, demonstrable performance in intended use should supersede general compliance with standard tests like NSF 49.
Conclusions:
The predominant piece of equipment used in viral and biological analysis has been the Class II Biosafety Cabinet specified by NSF/ANSI Standard 49.
This situation is changing. While tissue culture applications were the original design influencers for Standard 49, there are many newer research and production applications present today with different containment requirements and air flow designs. There is not any rectangular cuboid that can provide effective containment for all automated processes, gene splicing, lyophilizing, analytical weighing, medium scale process grinding, viral analysis, or antiviral syntheses.
New demands in medical research, pharma research, and pharma production require more custom equipment with highly specialized interior spaces and connectivity. These containment devices have unique performance requirements which need to be customer-defined and verified at the point of manufacture with customized testing.
Such a diverse group of urgently required containment equipment can never, ever, be reliably safety-certified using any single, general standard like NSF 49! Luckily, there is already a framework for evaluating purpose-built equipment: Assessing Particle Containment Performance of Pharmaceutical Equipment. The use of this framework requires manufacturers to know how to properly listen to their customers, devise a solution, and verify performance through focused testing.
Eventually, we may see sets of frequently ordered custom products arise from our current chaotic situation. Such product sets will invite classification, standardization, and common performance criteria.
So be it! But we are not there yet! We are at a place where each unique new product can be shown to do its job safely! Let us go with that for now. Later, we will be able to develop groups of products with common purposes and attributes. Such groups will operationally define the new products for which specific standards can be written.
References:
- Wedum, AG, 1969, The Detrick experience as a guide to the probable efficacy of P4 microbiological containment facilities for studies on microbial recombinant DNA molecules, J Am Biol Assoc; 1, 7.25
- NSF International, Wikipedia, 8/24/20; https://en.wikipedia.org/wiki/NSF_International
- NSF Standard #49, Labconco Corporation, 2020
- NSF Mission and History, 2020, https://www.nsf.org/about-nsf/mission-history
- NSF/ANSI 49-2016. Biosafety Cabinetry: Design, Construction, Performance, and Field Certification, 2016, Joint Committee on Biosafety Cabinetry c/o NSF International, https://webstore.ansi.org/preview-pages/NSF/preview_NSF+ANSI+49-2016.pdf
- Certification of Class II biological safety cabinets, David C. Eagleson, Clean Rooms, April, 2008, http://www.tbipcp.co.za/downloads/training/files/buildingDesign_2012/References/Eagleson_2008_CleanRooms.pdf
“Class II cabinets are by far the most prevalent, and NSF 49 is specific to Class II BSCs. Therefore, this article will focus on Class II BSCs, and all subsequent uses of “BSC” are in respect to Class II BSCs.”
- The Scientist, July/August 2020, “How the Pharma Industry Pulled off the pivot, Diana Kwon, https://www.the-scientist.com/bio-business/how-the-pharma-industry-pulled-off-the-pivot-to-covid-19-67719
ISPE Good Practice Guide: Assessing the Particulate Containment Performance of Pharmaceutical Equipment Second Edition, the International Society for Pharmacoepidemiology, 2012, Abromovitz, Marc; Farris, John; Floura, Hari; Flueckiger, Andreas; Marshall, Peter, CEng, MI-ChemE; Meiners, Matthew; Petroka, George S., CIH, CSP; Sussman, Robert; Taylor, Barbara; Wood, James P.
- https://flowsciences.com/evaluating-a-fume-hood-for-containment/
- Biosafety in Microbiological and Biomedical Laboratories, 5th Edition, S. Department of Health and Human Services, Public Health Service Centers for Disease Control and Prevention, National Institutes of Health HHS Publication No. (CDC) 21-1112, Revised December 2009
- Evaluating a Chemical Fume Hood for Containment of Solids, Liquids, and Vapors Using ASHRAE 110, HAM, and ISPE Methods, Goodman and Haugen, 2018, https://flowsciences.com/evaluating-a-fume-hood-for-containment/
- Nuaire History, Nu Aire, Inc., Plymouth, MN., https://www.nuaire.com/about-us/history,
- Biological Safety Cabinets, April 1991, Kruse, Puckett, and Richardson, Clinical Microbiological Reviews, pp.207 – 241.
14. Characterization of a human ovarian carcinoma cell line (NIH: OVCAR-3) with androgen and estrogen receptors, TC Hamilton, RC
Young, WM McKoy, KR Grotzinger, Nov.1983, Cancer Research
15. Report From the National Institute on Aging NIH Support of Cellular Aging Research: The Human Diploid “Fibroblast” Model,
Donald G. Murphy, PhD, March 1976, Journal of Gerontology
NIH: An account of Research in Its Laboratories and Clinics, DeWitt Stetten, Jr., Office of the Director National Institutes of Health, 1984, Academic Press Inc.
NIH-03-112C: Specification. Class II, type1 safety cabinet, 1974, National Institutes of Health, Bethesda, Md.
- https://www.linkedin.com/company/flow-sciences-inc-/

